
During exercise, a person may give off 185 kcal of heat in 25 min by evaporation of water at from the skin. How much water has been lost? [Hint: See page 399.]
In order to watch this solution you need to have a subscription.
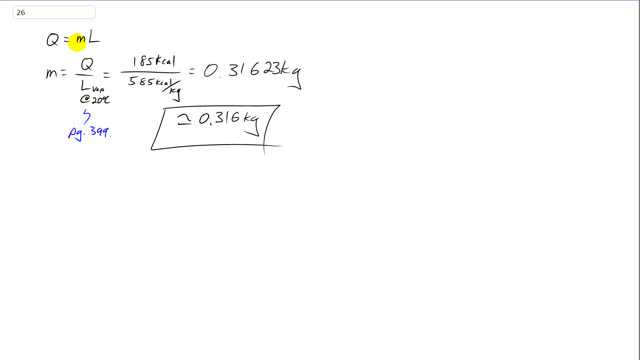
This is Giancoli Answers with Mr. Dychko. Q is the amount of heat given up due to the vaporization of the water as sweat leaving the skin, and that heat equals the mass of water times the latent heat of vaporization. But this L is going to be a little bit different than what you're used to, it's not the one in that table, instead, it's in the text on page 399 because it's the latent heat of vaporization at 20 degrees Celsius. Because water can change from liquid to gas at this temperature 20 below its boiling point, just happens to go really slowly. But it's important because it's the main mechanism by which the body cools itself down. So, divide both sides by L here to solve for m. And we have the total energy dissipated through the sweat is 185 kilocalories divided by 585 kilocalories per kilogram, latent heat of vaporization for water at 20 degrees Celsius. And that's 0.316 kilograms of water. And this gives you an idea of why it's important to drink water while exercising, because this is about 316 milliliters of water that's just disappeared out of your body in 25 minutes..
I am very confused about how the water can evaporate in room temperature. I know that the water cannot reach 100 celsius from our skin but how can water just evaporate in room temp?
Hi chaegyunkang, nice question! It turns out that temperature is an average kinetic energy of the particles of the substance. Water at has molecules with an average kinetic energy sufficient to turn into gas, so many of them do really quickly, and we observe this as boiling. Water at, say, also has some molecules with enough kinetic energy to turn into gas, but not very many, so we don't see anything special such as bubbles or steam. Nevertheless those few molecules with enough energy to do so turn into gas. This is what's responsible for evaporation, and eventually the luke warm water will all turn to gas for this reason.
Even ice evaporates! A full ice cube tray in your freezer will eventually have much smaller ice cubes after months since some of the molecules have enough kinetic energy to turn into a gas, even though the average kinetic energy is such that the molecules are in the ice phase.
All the best,
Mr. Dychko