
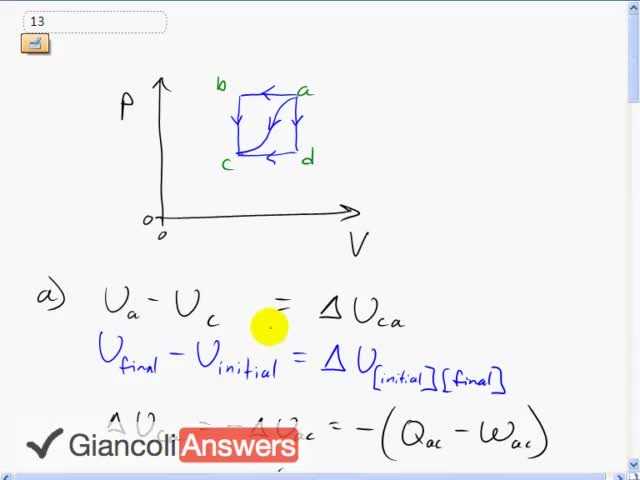
b)
c)
d)
In order to watch this solution you need to have a subscription.
Hi,
May you go over part a? I don't get why there's a negative sign in front of delta Uac and negative sign in front of the parenthesis. Thank you.
Hi LNY, thanks for the question about the negative signs. Negatives are definitely a source of confusion with Thermodynamics, often when talking about the work done by vs. on a gas... but I digress. This question asks for the difference in internal energy . This is a process where point a is the final state, and c is the initial state. This follows the usual convention as in other topics (change in velocity, change in momentum, etc.) where you state final minus initial. In thermodynamics, , and it's in the subscripts for the delta notation where I think the confusion begins, because the initial state is listed first in the subscript, whereas the final is listed second. A better way to write it is with an arrow in the subscripts , and perhaps I should have mentioned the arrow in the video.
To get to the point, the data we're given in the question is for a process starting at a and ending at c, both for the heat and the work. This means we can calculate . However, part a) of the question is asking for , which is in the opposite direction. Internal energy is a state variable, which means it doesn't matter what path is taken to get between two different points, much like the height of a book above the floor is a state variable since it doesn't matter whether it was lifted straight up or lifted along a curved path. In the book analogy, friction is not a state variable since the work done by friction does depend on the path, much like heat added to the gas and work done by the gas are not state variables in the topic of thermodynamics here. Regardless of what Q or W are, the change in internal energy is always the same between two specific points in the PV diagram. And now, here's the one sentence that might have sufficed, but I wanted some preamble since without a conversation with you it's hard to tell how much preamble is needed:
since these processes are in opposite directions between the same two points. The parenthesis that follows surrounds the replacement for with and the negative just stays where it is.
OK, that was two sentences, but here's one: These processes are between the same two points in opposite directions, so the change in internal energy in one direction is equal to the negative of the change in internal energy in the other direction.
Hope this helps!
Mr. Dychko
Very well explained. I had to read it couple times over. Thank you!