Problem 38
Q
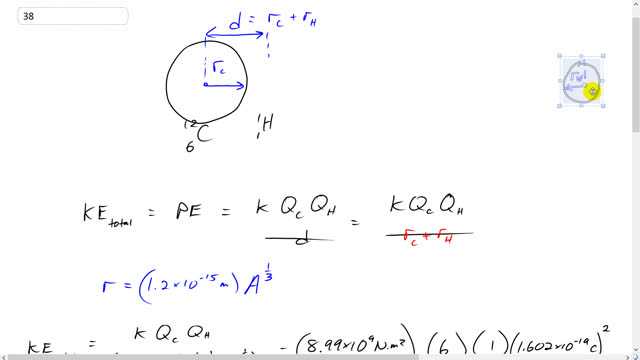
- Give the ratio of the energy needed for the first reaction of the carbon cycle to the energy needed for a deuterium–tritium reaction (Example 31–9).
- If a deuterium–tritium reaction actually requires a temperature , estimate the temperature needed for the first carbon-cycle reaction.
A
- . The carbon reaction requires 5 times as much initial kinetic energy as the deuterium-tritium reaction.
In order to watch this solution you need to have a subscription.
Giancoli Answers, including solutions and videos, is copyright © 2009-2026 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.
