Problem 52
Q
Radon gas, , is considered a serious health hazard (see discussion in text). It decays by -emission.
- What is the daughter nucleus?
- Is the daughter nucleus stable or radioactive? If the latter, how does it decay, and what is its half-life? (See Fig. 30–11.)
- Is the daughter nucleus also a noble gas, or is it chemically reactive?
- Suppose 1.4 ng of seeps into a basement. What will be its activity? If the basement is then sealed, what will be the activity 1 month later?
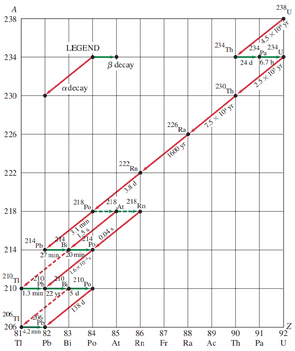
Figure 30-11 Decay series beginning with . Nuclei in the series are specified by a dot representing and values. Half-lives are given in seconds (s), minutes (min), hours (h), days (d), or years (yr). Note that a horizontal arrow represents decay ( does not change), whereas a diagonal line represents decay ( changes by 4, changes by 2). For the four nuclides shown that can decay by both and decay, the more prominent decay (in these four cases, >99.9%) is shown as a solid arrow and the less common decay (<0.1%) as a dashed arrow.
A
- There is also a bit of decay.
- Chemically reactive. It's in the same group as Oxygen on the periodic table.
- initial: , after one month:
In order to watch this solution you need to have a subscription.
Giancoli Answers, including solutions and videos, is copyright © 2009-2026 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.
