
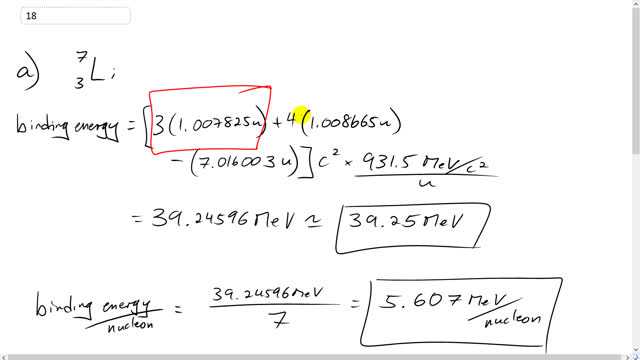
Calculate the total binding energy, and the binding energy per nucleon, for
- ,
- .
In order to watch this solution you need to have a subscription.
Is this incorrect because the binding energy is the difference between the protons and neutrons added together and the atomic mass?
Hi eshhann,
Thank you for the question. I think you have the right idea - you're quite right that the binding energy is the total mass of free protons and free neutrons, minus the mass of those protons and neutrons bound in the nucleus. However, keep in mind that atomic mass includes the electrons surrounding the nucleus. An atom includes as many electrons as protons, otherwise it's called an ion. The data we have is for atomic masses, not the mass of the protons and neutrons bound in the nucleus, but rather we have the bound protons and neutrons and electrons surrounding them. With lithium as an example, the atomic mass includes the mass of three electrons. There are two ways to deal with this issue. Approach 1: (I think this is would work for the way you're thinking about it) we can find the total mass of free protons and free neutrons and then subtract the atomic mass of lithium minus the mass of three electrons. The atomic mass of the lithium needs to be reduced by the mass of the electrons surrounding the atom in order to get the mass of the nucleus. This would get the correct answer. The approach I use in the video, however, is to use the atomic mass of hydrogen, rather than the mass of a free proton, since that adds three electron masses (one for each hydrogen atom) to compensate for the electrons surrounding the lithium atom.
All the best,
Shaun