
Giancoli's Physics: Principles with Applications, 7th Edition
30
Nuclear Physics and Radioactivity
Change chapter30-1: Nuclear Properties
30-2: Binding Energy
30-3 to 30-7: Radioactive Decay
30-8 to 30-11: Half-Life, Decay Rates, Decay Series, Dating
Question by Giancoli, Douglas C., Physics: Principles with Applications, 7th Ed., ©2014, Reprinted by permission of Pearson Education Inc., New York.
Problem 53
Q
Rubidium-strontium dating. The rubidium isotope , a emitter with a half-life of , is used to determine the age of rocks and fossils. Rocks containing fossils of ancient animals contain a ratio of to of 0.0260. Assuming that there was no present when the rocks were formed, estimate the age of these fossils.
A
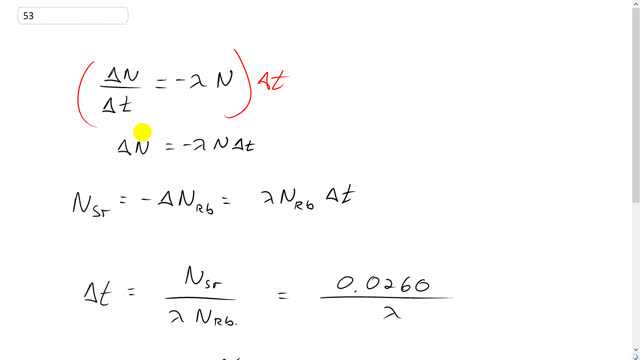
In order to watch this solution you need to have a subscription.
Giancoli Answers, including solutions and videos, is copyright © 2009-2025 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.