Problem 12
Q
How high would the level be in an alcohol barometer at normal atmospheric pressure?
A
In order to watch this solution you need to have a subscription.
VIDEO TRANSCRIPT
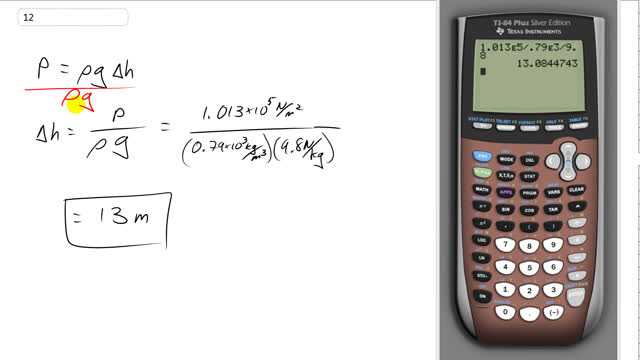
This is Giancoli Answers with Mr. Dychko. With a barometer, the pressure that it measures is the density of the fluid inside times g times the height of the fluid and we can solve for the height by dividing both sides by density and dividing both sides by g. So the height of the alcohol column would be the atmospheric pressure 1.013 times 10 to the 5 newtons per square meter divided by the density of alcohol— 0.79 times 10 to the 3 kilograms per cubic meter— times acceleration due to gravity or gravitational field strength— 9.8 newtons per kilogram— and that gives 13 meters will be the height of the alcohol column.
Giancoli Answers, including solutions and videos, is copyright © 2009-2026 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.
