How many helium-filled balloons would it take to lift a person? Assume the person has a mass of 72 kg and that each helium-filled balloon is spherical with a diameter of 33 cm.
In order to watch this solution you need to have a subscription.
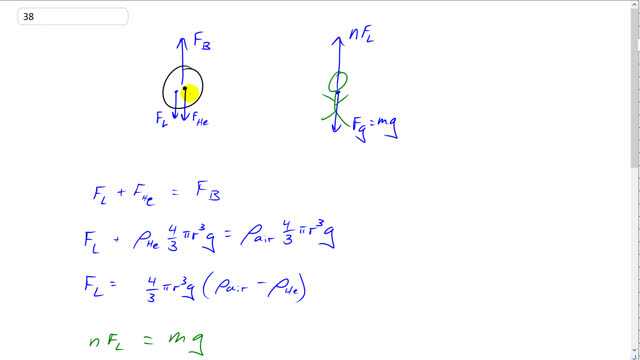
This is Giancoli Answers with Mr. Dychko. Let's consider just a single balloon for a moment: we have this buoyant force equal to the weight of air displaced by the balloon that's going upwards and then we have the weight of the helium downwards and we also have this load that's pulling the balloon down and this load is at a maximum when the load plus the weight of the helium equals the buoyant force upwards. So that's considering one balloon and this solves for the maximum load force that could be exerted downwards on a single balloon. When we consider a person, they are gonna have n times this maximum load force so the number of balloons, n, times F L—that's gonna be the force upwards— and then that's gonna have to equal their weight downwards, mg. So we have the weight of the helium is the mass of helium which is helium's density times by the volume which is four-third's πr cubed because this balloon is a sphere and that's times by g and that equals the density of air displaced by the balloon times by this same volume— the volume of air displaced by the balloon is the volume of the balloon— times by g and so the maximum load force after you subtract this term from both sides is gonna be four-third's πr cubed g which is a common factor between these two terms multiplied by ρ air minus ρ helium— density of air minus density of helium— and then that we'll substitute down here. So we have n times F L equals mg so now we are looking at this picture of a person and we'll solve for n by dividing both sides by F L and we have the number of balloons is mg divided by F L which is mg divided by four-third's πr cubed g times the difference in the densities between air and helium and cleaning that up a bit, the g's cancel and this 3 goes on the top we have 3m over 4πr cubed difference in their densities and then we plug in some numbers. And we have 3 times 72 kilograms divided by 4π times this diameter divided by 2 to get radius and then converting the centimeters into meters by multiplying 33 by 10 to the minus 2, cube that radius times by 1.29 kilograms per cubic meter—density of air— minus 0.179 kilograms per cubic meter— density of helium— and you get about 3400 balloons would be needed to lift this person.
