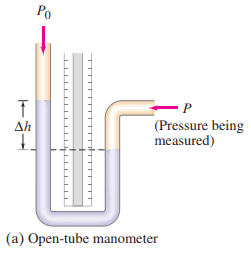
An open-tube mercury manometer is used to measure the pressure in an oxygen tank. When the atmospheric pressure is 1040 mbar, what is the absolute pressure (in Pa) in the tank if the height of the mercury in the open tube is
- 18.5 cm higher,
- 5.6 cm lower, than the mercury in the tube connected to the tank? See Fig. 10–7a.
In order to watch this solution you need to have a subscription.
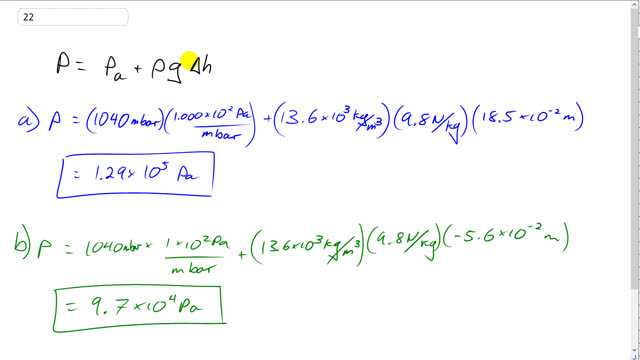
This is Giancoli Answers with Mr. Dychko. Absolute pressure in an open tube monomer is atmospheric pressure plus the density of the fluid in the monomer times g times the height by which the fluid in the open end of the monomer is higher than the fluid in the end that's connected to whatever thing is having its pressure measured. So we have the pressure in the first case is 1040 millibar times 1.00 times 10 to the 2 pascals per millibar— that's atmospheric pressure— plus this additional pressure due to the column fluid there, mercury, 13.6 times 10 to the 3 kilograms per meter cubed for mercury times 9.8 newtons per kilogram times 18.5 times 10 to the minus 2 meters— that's centimeters converted into meters— and we get 1.29 times 10 to the 5 pascals must be the absolute pressure. And then when we have the column of... or the height difference in the monomer is 5.6 centimeters lower it's the same numbers except we have negative 5.6 times 10 to the minus 2 meters for the height here and this gives us a pressure lower than atmospheric pressure: 9.7 times 10 to the 4 pascals.
In the video, you wrote 1.29 * 10^5 Pa, but in the main answer section, you wrote 1.20 * 10^5 Pa. Merely for clarity, can you correct the second answer?
Thank you very much for spotting that rdattafl. I've updated the quick answer.
