
The current in an electromagnet connected to a 240-V line is 21.5 A. At what rate must cooling water pass over the coils for the water temperature to rise no more than ?
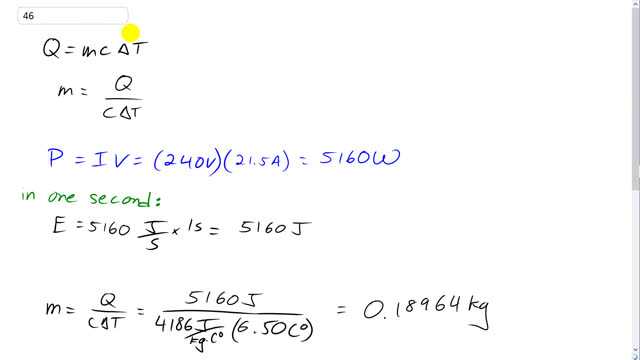
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The amount of heat that is absorbed by some water equals the mass of water, times the specific heat of water, times its change in temperature. And we can solve this for mass by dividing both sides by C Delta T. And we get mass of water is: the amount of heat absorbed, divided by its specific heat, times the change in its temperature. So, the power output of this electromagnet is the current going through it of 21 and a half amps, multiplied by 240 volts, which is 5160 watts. And, we're going to consider one second, and we're gonna figure out how much energy is absorbed by the water in one second, and that energy is gonna be the Q in this formula here. And, since we considered one second, then, we'll be able to use this formula to figure out the amount of mass required per second, which gives us the flow rate, mass per second. So, the energy in one second is 5160 watts, but the units watts is a shorthand way of saying Joules per second, and we multiply that by one second and we get 5160 Joules of energy ha s to be absorbed by the water, because that's the amount of energy output by the electromagnet in one second. And so the mass of water required will be that 5160 Joules, divided by the specific heat of water, 4186 Joules per kilogram-Celsius degree, times by the change in temperature of the water, which is allowed at a maximum of 6.50 Celsius degrees. And that's .18964 kilograms, and we can convert that into liters, 'cause typically, flow rates are given in liters per second. So, we have density of water is mass divided by its volume, and we can solve for V by multiplying both sides by V over rho. And so we have V is mass divided by density. So that's .18964 kilograms, divided by one kilogram per liter, which is the same numerical number in liters. So, the flow rate is .190 after you round this to three significant figures, liters per second because it's all came about by assuming one second.