
Giancoli's Physics: Principles with Applications, 7th Edition
29
Molecules and Solids
Change chapter29-1 to 29-3: Molecular Bonds
29-4: Molecular Spectra
29-5: Bonding in Solids
29-7: Band Theory of Solids
29-8: Semiconductors and Doping
29-9: Diodes
29-10: Transistors
Question by Giancoli, Douglas C., Physics: Principles with Applications, 7th Ed., ©2014, Reprinted by permission of Pearson Education Inc., New York.
Problem 10
Q
The equilibrium separation of H atoms in the molecule is 0.074 nm (Fig. 29–8). Calculate the energies and wavelengths of photons for the rotational transitions
- l = 1 to l = 0,
- l = 2 to l = 1, and
- l = 3 to l = 2.
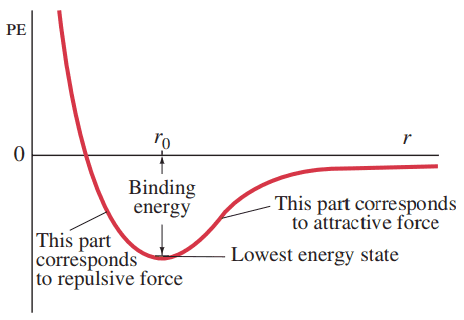
Figure 29-8 Potential-energy diagram for the molecule; is the separation of the two H atoms. The binding energy (the energy difference between PE = 0 and the lowest energy state near the bottom of the well) is 4.5 eV, and .
A
Giancoli Answers, including solutions and videos, is copyright © 2009-2025 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.