
Giancoli's Physics: Principles with Applications, 7th Edition
29
Molecules and Solids
Change chapter29-1 to 29-3: Molecular Bonds
29-4: Molecular Spectra
29-5: Bonding in Solids
29-7: Band Theory of Solids
29-8: Semiconductors and Doping
29-9: Diodes
29-10: Transistors
Question by Giancoli, Douglas C., Physics: Principles with Applications, 7th Ed., ©2014, Reprinted by permission of Pearson Education Inc., New York.
Problem 22
Q
We saw that there are 2N possible electron states in the 3s band of Na, where N is the total number of atoms. How many possible electron states are there in the
- 2s band,
- 2p band, and
- 3p band?
- State a general formula for the total number of possible states in any given electron band.
A
- 2N
- 6N
- 6N
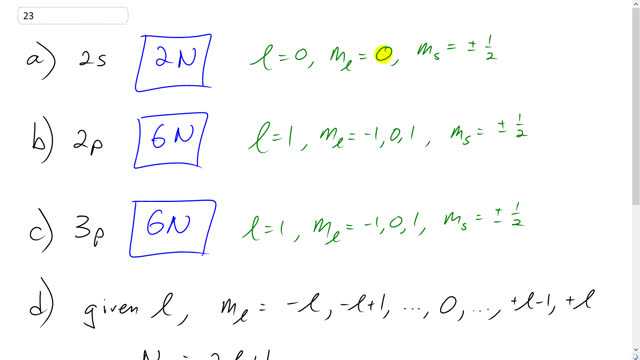
In order to watch this solution you need to have a subscription.
Giancoli Answers, including solutions and videos, is copyright © 2009-2025 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.