
An oxygen molecule consists of two oxygen atoms whose total mass is and whose moment of inertia about an axis perpendicular to the line joining the two atoms, midway between them, is . From these data, estimate the effective distance between the atoms.
In order to watch this solution you need to have a subscription.
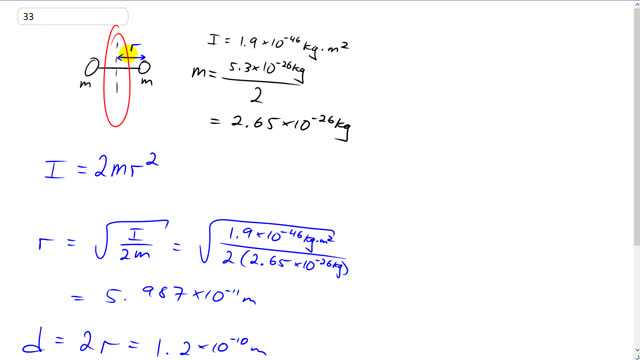
This is Giancoli Answers with Mr. Dychko. The moment of inertia for this oxygen molecule is 1.9 times 10 to the negative 46 kilogram meter squared and that is about this axis of rotation midway between the two atoms. And the mass of each atom is the total mass of the molecule divided by 2 and we end up with 2.65 times 10 to the minus 26 kilograms mass for each of the oxygen atoms that compose this molecule of O 2. So the moment of inertia is 2 times the moment of inertia for a single particle because there's two particles. So moment of inertia is 2 times mR squared and we can solve this for R by dividing both sides by 2m and then take the square root of both sides and we end up with r is the moment of inertia divided by 2m square rooted. So that's 1.9 times 10 to the negative 46 kilogram meter squared over 2 times 2.65 times 10 to the negative 26 kilograms square rooted and then multiply that by 2 to get the total distance between the atoms. So d is 2 times r and that gives about 0.12 nanometers if you write this with a prefix 'nano.' So a nanometer is 10 to the negative 9 and so 1.2 times 10 to the minus 10, you could, you know, multiply this part by 10 and then divide this part by 10 to compensate so that in the end you are doing nothing but multiplying by 1 and you end up with 1.2 divided by 10 is 0.12 and 10 to the negative 10 times 10 is 10 to the negative 9 which can be substituted with nanometers.