
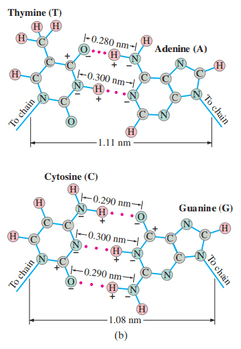
The two strands of the helix-shaped DNA molecule are held together by electrostatic forces as shown in Fig. 16–39. Assume that the net average charge (due to electron sharing) indicated on H and N atoms has magnitude 0.2e and on the indicated C and O atoms is 0.4e. Assume also that atoms on each molecule are separated by . Estimate the net force between
- a thymine and an adenine; and
- a cytosine and a guanine. For each bond (red dots) consider only the three atoms in a line (two atoms on one molecule, one atom on the other).
- Estimate the total force for a DNA molecule containing pairs of such molecules. Assume half are A–T pairs and half are C–G pairs.
In order to watch this solution you need to have a subscription.
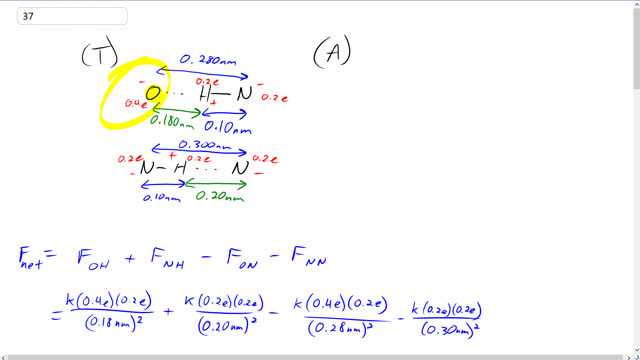
This is Giancoli Answers with Mr. Dychko. So I've drawn the parts of the nucleotide basis that are most strongly involved in their electrostatic bonding. For thiamine, we have this oxygen which has the charge of 0.4 times the elementary charge, E, and it's going to be negative 0.4 E because oxygen is a highly electronegative element. So it's doing some unfair sharing with the carbon that's nearby, and the carbon is losing some of its thiamine electron, and the electron is spending more time in the oxygen here. But this carbon is further away so it's not part of our picture. And on the adenine side, we have a hydrogen bonded to nitrogen, and hydrogen will have a charge of positive 0.2e and nitrogen will have a charge of negative 0.2e. Nitrogen being negative because it's more electronegative than hydrogen, that it can pull electron that hydrogen has away from it for some of the time. So the distance between atoms on a molecule is 0.1 nanometers as we're told. That's the distance between the H and the N. From the picture in Figure 16-39, the separation between the N and the O is 0.28 nanometers, which makes this distance between the O and the H, 0.28 minus 0.1 which is 0.18 nanometers. And down here, it's almost a mere image except we have a nitrogen here instead of an oxygen. The distance between the nitrogen is 0.3 nanometers. And this being 0.1 makes 0.2 nanometers distance here. The net force is the question. Let's find the net force on the thiamine. Forces to the right are positive and forces to the left are negative. There. Between this oxygen and this hydrogen, we have a positive force to the right because they're attracting. That's reflected by this term. That's gonna be k times the charge on the oxygen of 0.4e times the charge in the hydrogen of 0.2e and divided by the distance between of 0.18 nanometers squared. We'll convert this nanometers into meters later, I'd be writing times 10 the minus 9 too many times here so I didn't bother writing it yet but we'll do that later on. Then another attractive force is between this nitrogen on the adenine and this hydrogen on the thiamine. That's gonna be k times 0.2e charge in the nitrogen, times 0.2e charge in the hydrogen, divided by the distance between them of 0.2 nanometers squared. Pushing to the left on this thiamine is the repulsion between the oxygen and the nitrogen, so that's resulting on a force this way, so we put a minus sign because the force is to the left, and that's gonna be k times 0.4e times 0.2e in the nitrogen, divided by their distance between them is 0.28 nanometers squared. Then, there's a repulsive force between these nitrogens, and so that results in a force to the left of the thiamine. And that's going to be 0.2e times 0.2e divided by 0.3 nanometers between them, squared. There we go. Those are all the forces that are important. Now it's calculation. We factor out the e squared and the k from each term, and we're left with just these, 0.4 times 0.2 in the first term, divided by 0.18 nanometers squared, and then 0.2 squared over 0.2 nanometers squared, 0.4 times 0.2 divided by 0.28 nanometers squared, and 0.2 squared divided by 0.3 nanometers squared. Inside here, we're left with units of one over nanometers squared which we convert into meters square, but there's number here. I just put this one times 10 to minus 9 meters per nanometer squared in order to convert the nanometers which ends up down here into meter squared. We have nine times 10 to the nine, which is Coulumb's constant, times 1.6 times 10 to minus 19 Coulombs squared, and then divided by this conversion factor and times by-- When you plug all these into your calculator, it leads to the number of 2.00428. It has units of one over nanometer squared, but I didn't bother writing that. We've already talked about the units. Then we end up with 4.6 times 10 to the negative 10 Newtons as the net force to the right on the thiamine. It's also the net force on the adenine to the left because there's a Newton's third law of pairs. So the adenine exerts on the thiamine is of equal magnitude but opposite direction to the force of thiamine exerts on the adenine. Then there's cysteine and guanine. There are three pairs to consider here. Let's find the net force on the cysteine nucleotide base. We have some attraction between oxygen and hydrogen here, as well as here. We can go two times the force between oxygen and hydrogen. The distances are the same according to that picture, 0.29 nanometers in each case. I guess I should put this into down here. It's a long question, isn't it? We have a total distance between the nitrogen and oxygen is 0.29 nanometers, which makes the distance between the oxygen to the hydrogen 0.19 nanometers. Since we're told the distance between atoms, such as between the hydrogen and nitrogen, is 0.1 nanometers, which leaves this leftover as 0.29 minus 0.1 which is 0.19 nanometers. Then, that's to the right on the cysteine so that's positive. Then we have the attraction between the nitrogen and hydrogen here, and that's gonna be 0.2 times e, which is the charge in each of them. We square that and divided by 0.2 nanometers, the distance between the nitrogen and the hydrogen here, and since it is between nitrogen and nitrogen is 0.3 nanometers in total, and then take away the 0.1 between the hydrogen and nitrogen leaving us with 0.2 between the nitrogen and the hydrogen. This nitrogen and the hydrogen. Two repulsion terms which are pushing the cysteine to the left, we have two times the force between this nitrogen and this oxygen as well as this nitrogen and this oxygen, and that's 2k times 0.2e times 0.4e over 0.29 nanometers squared. Then the repulsion between this nitrogen and this nitrogen which is 0.2e squared, it should be 0.2 there, 0.2e squared over 0.3 nanometers. This is between the two nitrogens squared. Then same process before, factor out the ke squared, and also introduce this conversion term to convert the nanometers squared and the denominator in 10 meters squared in the denominator. Work all this out and you end up with 3.08519 times by elementary charge, divided by the conversion factor, two times by Coulomb's constant, and you get 7.1 times 10 to the minus 10 neutrons as the net force, to the right on the cysteine and also the net force to the left of the guanine. Then the total force if you had 10 to the five total base pairs, half of which are adenine-thiamine pairs and half of which are cysteine-guanine pairs, then the total force is gonna be the net force between adenine and thiamine times to the five over two, because there's 10 to the five divided by two pairs of them. Then there's 10 to the five over two pairs of cysteine-guanine, so take that net force and multiply it by that. So we have 4.618 times 10 to the minus 10 net force between adenine and thiamine times 10 to the five over two, plus 7.108 times 10 to the minus 10 Newtons between the cysteine and guanine times 10 to the five over two. And we end up with six times 10 to the negative five Newtons as the total force between an entire DNA molecule. There we go.