
What is the total charge of all the electrons in a 12-kg bar of gold? What is the net charge of the bar? (Gold has 79 electrons per atom and an atomic mass of 197 u.)
In order to watch this solution you need to have a subscription.
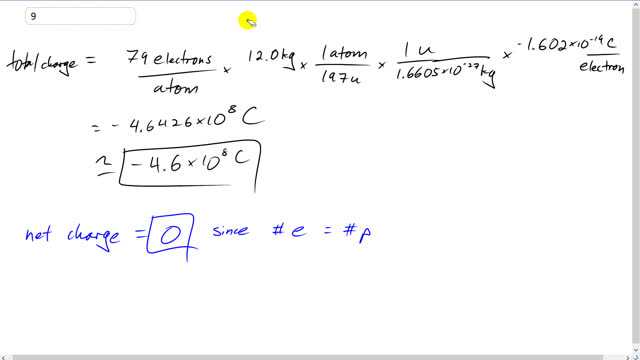
This is Giancoli Answers with Mr. Dychko. So to answer this question you have to think of a bunch of conversion factors to get the units that you eventually want. We know that we want a number of electrons because we can convert that into Coulombs pretty easily by multiplying by elementary charge in coulombs per electron and so we only have 79 electrons per atom of gold and we need to multiply that by the number of atoms and we're given 12.0 kg to work with. So want to convert these kilograms into number of atoms. So we have 12 kilograms times well we don't know how many atoms there are per kg. We multiply by one atom for every 197 atomic mass units. We know that there's the atomic mass of the gold is 197. So there's one atom for every 197 atomic mass units. We can convert that into kilograms by multiplying by one atomic mass unit for every 1.6605 times ten to the minus 27 kilogram. So kilogram cancelling here and the atoms are canceling and the electrons are canceling leaving us with coulombs. So mission accomplished. So 79 electrons per atom times 12.8 kilograms times when atom for every 197 atomic mass units times this to convert the atomic mass units into Kilograms times 1.602 times ten to the minus nineteen coulombs per electron that's negative because we're dealing with electrons here and that gives negative 4.6 times ten to the eight coulombs is a total charge of electrons on 12 kilograms of gold. The net charge will be zero because there's an equal number of electrons as there are protons.
no sense was made
agree. this is A piece of shit.
Where did the amu in kg come from?