- What is the electric potential away from a proton (charge +e)?
- What is the electric potential energy of a system that consists of two protons apart—as might occur inside a typical nucleus?
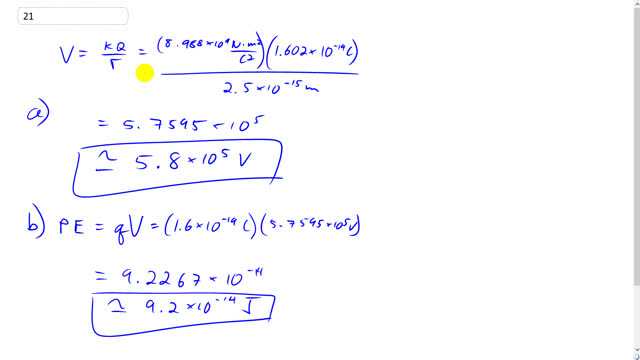
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The electric potential due to the proton, when you're 2.5 times 10 to the minus 15 meters away is the Coulomb’s constant times the charge divided by the distance. So we have 8.98 times 10 to the 9 Newton meters squared, per Coulomb squared, times the charge on a proton, which is 1.602 times 10 to the minus 19 Coulombs, divided by this distance, and you get about 5.8 times 10 to the five volts. That's the potential difference between zero and infinity versus two and a half femtometers. And the potential energy of a system with two protons separated by this distance, is gonna be k or q times the electric potential. So one of the protons is gonna experience an electric potential due to the other proton, and so we multiply the charge on one of them by the potential due to the other so its 1.6 times 10 to the minus 19 Coulombs times the answer for part a 5.7595 times 10 to the 5 volts. This gives 9.2 times 10 to the minus 14 Joules, is the potential energy of that two proton system.
