
In order to watch this solution you need to have a subscription.
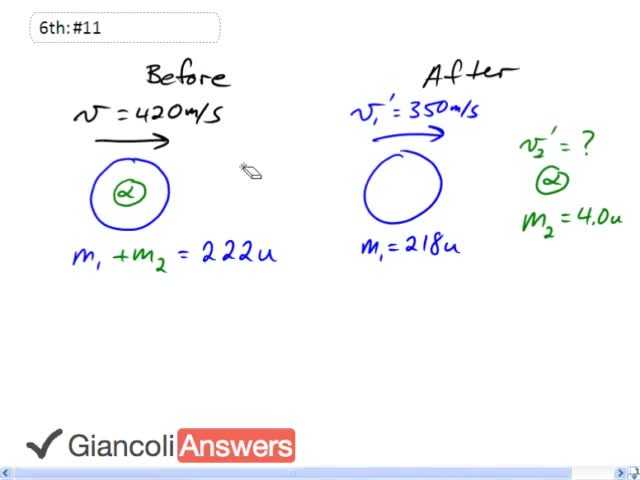
We have this atomic nucleus moving with initial velocity of four hundred and twenty meters per second and it emits an alpha particle which is actually a helium nucleus with two protons and two neutrons in it that’s why it has a mass of four atomic units, each particle in the nucleus has one atomic mass unit. So the question is what's the speed of that alpha particle? We’ll use conservation of momentum to answer that. So we’ll say the total mass of this thing at the beginning which is two hundred and twenty two, or we could say it’s: ‘m1’ plus ‘m2’, ‘m1’ being the mass of the nucleus after the alpha particle is gone, and ‘m2’ being the alpha particle mass. That initial momentum before the particle is emitted is equal to the total momentum after it’s emitted, so we have: ‘m1’ times ‘v1`’ plus ‘m2’ times ‘v2`’. So let’s solve this for ‘v2`’: ‘m2’ times ‘v2`’ equals ‘m1’ plus ‘m2’ times ‘v’ minus ‘m1’ times ‘v1`’, dividing both sides by ‘m2’, ‘v2`’ is ‘m1’ plus ‘m2’ times ‘v’ minus ‘m1’ times ‘v1`’ all over ‘m2’. Substituting in numbers, we get our answer: two hundred and eighteen atomic mass units plus four atomic mass units times four hundred and twenty meters per second minus two hundred and eighteen atomic mass units times three hundred and fifty meters per second all divided by four atomic mass units which makes four point two times ten raised to power three meters per second. That is the speed of the alpha particle. We didn’t need to change units to kilograms because when you add the two masses together you’ll end up with units of u atomic mass units, and that’ll be u times meters per second. So the units from this bit are u multiplied by meters pr second, and when we do the subtraction on the numerator of this whole fraction the units will also be u times meters per second, when you do the division the u’s cancel leaving us with meters per second. So it’s unnecessary to convert to meters per second, though if you wanted to you could. So one atomic mass unit is one point six six zero five times ten raised to minus twenty seven kilograms.