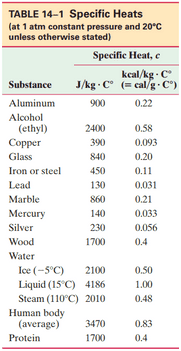
Samples of copper, aluminum, and water experience the same temperature rise when they absorb the same amount of heat. What is the ratio of their masses? [Hint: See Table 14–1.]
In order to watch this solution you need to have a subscription.
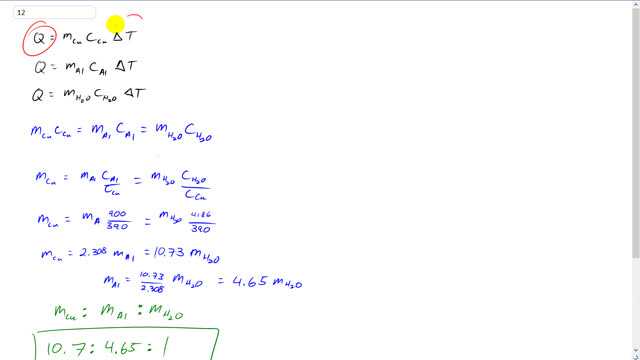
This is Giancoli Answers with Mr. Dychko. Since the heat gained by each material is the same and the change in temperature for each material is the same. When we write the heat equation, Q does not need a subscript and ΔT does not need a subscript because it's the same for each substance. But we will put a subscript on the mass of copper in one case times the specific heat capacity of copper and times mass of aluminum specific heat capacity of aluminum. And we have a subscript water for the mass here and water for the specific heat capacity here. And since each to these things are equal to Q, they're all equal to each other, each these things is equal to each other, and the Δ temperature is going to cancel for each one. So, we can write this line here. And so this says the mass times the heat capacity of each substance is the same. And we'll divide each thing by the material that has the smallest heat capacity to get ratios of masses here. So, this when you divide by the specific capacity of copper you get mass of copper equals mass of aluminum times the specific heat capacity of aluminum divided by that of copper. And then mass of water times that ratio there. And then substituting in 900 joules per kilogram Celsius degree, specific capacity of aluminum divided by that of copper, 390, gives 2.308. And then 4,186, specific heat capacity of water, divided by, 390, capacity for copper, gives 10.73. And so this means the mass of copper is 10.7 times the mass of water. And then if we want to express everything in terms of or as multiples of the mass of water, because this is the smallest mass since it has the highest specific heat capacity. We'll write 10.7 parts copper for every 1 part water. And then the mass of aluminum in terms of the mass of water is going to be 10.73 divided by 2.308 which is 4.65. So, there's 4.65 times as much... The mass of aluminum is 4.65 times out of the mass of water. So, there's 4.65 parts of aluminum for every 1 part water. So, 10.7 to 4.65 to 1 is the mass of copper to the mass of aluminum to the mass of water.
