A 215-g sample of a substance is heated to and then plunged into a 105-g aluminum calorimeter cup containing 185 g of water and a 17-g glass thermometer at . The final temperature is . What is the specific heat of the substance? (Assume no water boils away.)
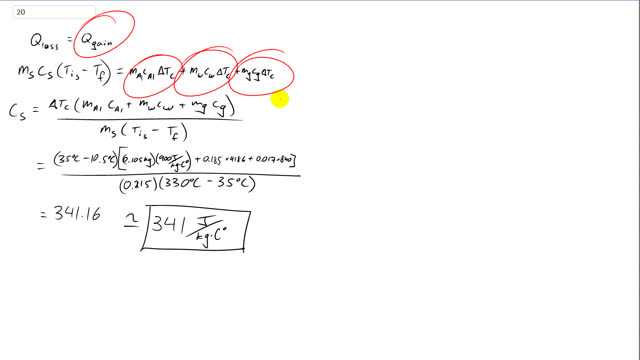
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The heat lost by this mystery substance has been equal the heat gained by this calorimeter cup and the water that it contains and also the glass thermometer that's inside of it. So, all of these things will be in equilibrium when the total heat lost by the substance equals the total heat gained by everything else. So, we have mass of aluminium times specific heat of aluminium times the change in temperature of the calorimeter plus mass of water times specific heat of water times the same change in temperature because the calorimeter, the water and the glass thermometer all start at the same temperature and they all have the same final temperature. So, they have the same change in temperature let's call it ΔTc for a change of temperature of the calorimeter and everything that's in it. Plus the mass of the glass times specific heat of glass times its change in temperature. So, a factor out the ΔTc and you get this line here, and then divide both sides by ms and change in temperature of this mystery substance and we can solve for the specific heat of this substance. So, it's changing temperature times all this stuff divided by mass of the substance times the initial temperature of the substance minus its final temperature. And we have initial minus final whenever we have heat lost. So, that's 35 degrees Celsius minus 10.5 degrees Celsius, and that's final temperature minus the initial temperature for the calorimeter and everything that was initially inside it. Times 0.105 kilograms mass of the calorimeter times 900 joules per kilogram Celsius degree, specific heat of aluminum plus 0.185 kilograms, mass of the water, times 4,186 joules per kilogram Celsius degree, specific heat of water. I'm not writing all the unit's here because there's so many numbers and units that it would take a long time to write. Plus 0.017 kilograms for the mass of the glass that thermometer’s made out of plus it or times 840 joules per kilogram Celsius degrees, specific heat of glass, divided by 0.21 of kilograms mass of this mystery substance times its initial temperature of 333 degrees Celsius minus this final temperature of 35 degrees Celsius. And so a specific heat must be 341 joules per kilogram Celsius degree.
