
The 1.20-kg head of a hammer has a speed of 7.5 m/s just before it strikes a nail (Fig. 14–17) and is brought to rest. Estimate the temperature rise of a 14-g iron nail generated by eight such hammer blows done in quick succession. Assume the nail absorbs all the energy.
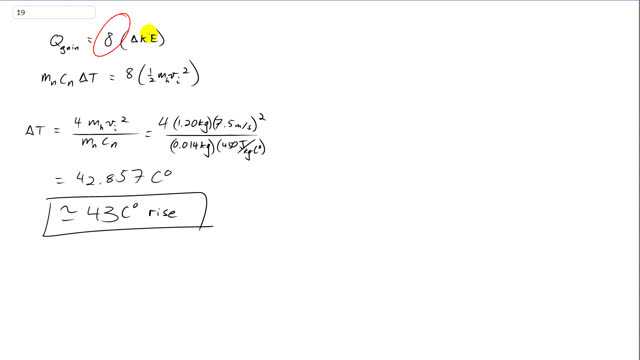
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. We assume that all the kinetic energy lost by the hammer is gained as heat in the nail. And the hammer is going to lose its kinetic energy 8 times because there's 8 swings. And the change in kinetic energy is 1/2 mass of the hammer times its initial speed squared minus 1/2 mass of the hammer times its final speed squared. And I guess I'm kind of writing it around backwards, usually it's final minus initial, but it's just the magnitude of the change that matters here. So... And... So, we plug in for the heat gained by the nail is going to be the mass of the nail times its specific heat times its change in temperature. That's gonna be 8 times 1/2 mass of the hammer times the hammer's initial speed squared. And we can solve for ΔT by dividing both sides by mass of the nail and it's also its specific heat. And 8 times 1/2 gives 4. So, we have the change in temperature is 4 times mass of the hammer times its initial speed squared divided by mass of the nail times the specific heat of the nail. So, that's 4 times 1.2 kilograms times 7.5 meters per second squared divided by 0.014 kilograms, mass of the nail, times 450 joules per kilogram Celsius degrees, specific heat of iron, which is what the nail is made out of. And that gives a 43 Celsius degree rise. And that does not mean that the nails temperature will be 43 degrees Celsius, instead it will be 43 Celsius degrees higher than whatever the nails initial temperature is, which we don't know. If it was room temperature of 20 degrees Celsius, then the final temperature of the nail would be 63 degrees for example. So, anyway. 43 Celsius degrees is how much the temperature will rise.