When a diver jumps into the ocean, water leaks into the gap region between the diver’s skin and her wetsuit, forming a water layer about 0.5 mm thick. Assuming the total surface area of the wetsuit covering the diver is about , and that ocean water enters the suit at and is warmed by the diver to skin temperature of , estimate how much energy (in units of candy bars = 300 kcal) is required by this heating process.
In order to watch this solution you need to have a subscription.
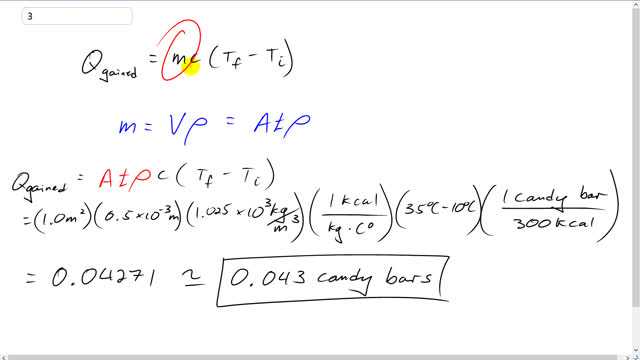
This is Giancoli Answers with Mr. Dychko. The heat gained by this water in the half millimeter boundary layer between the divers skin and their wetsuit is the mass of water that's in that boundary layer times the specific heat capacity of water, and this is ocean water by the way. And, although that has no bearing on the specific heat capacity but it does have a bearing on the mass because we're going to use the volume of that space between the skin and the wetsuit multiplied by the density of the water, and we'll have to use the density of ocean water there. And multiplied by the temperature difference, it goes from 10 degrees to a final temperature of 35 degrees Celsius. So, the mass is volume times density and the volume is the surface area of the wetsuit multiplied by the thickness of the boundary layer. And that's gonna, and then multiplied by the density of water, and, you know, kilograms per cubic meters. So, this is substituting back into the formula for the heat gained, it's going to be area times thickness times density times specific heat capacity times the change in temperature. So, that's 1 square meter times 0.5 times 10 to the minus 3 meters. Converting those millimeters into meters there by multiplying by 10 to the minus 3. And then times by 1.025 times 10 to the 3 kilograms per cubic meter density of ocean water times 1 kilocalorie to raise 1 kilogram of water 1 Celsius degree. And the kilograms are cancelling and while the cubic meters here already cancelled there. And we're left with kilocalorie per Celsius degree and then we multiply by the change in temperature, 35 minus 10. And then the Celsius degrees will cancel. And then 1 candy bar has 300 kilocalories. So, kilocalories will cancel now too. And we end up with 0.043 candy bars of energy are needed to heat the water in that boundary layer in the wetsuit.
I think specific heat capacity of 4.186 is missing in the calculation? Can you please clarify
Bonjour girondebordeaux92, thanks for the question. The 4.186 figure you're quoting is, with units, . Understanding units answers your question, since if you consult table 14-1 you'll notice that an alternative way to mention the specific heat of water is to write . It turns out that the "kcal" way of writing specific heat, as shown in the video, if just convenient in this case since the energy of the candy bar is giving in kcal.
All the best,
Mr. Dychko
