
Giancoli's Physics: Principles with Applications, 7th Edition
14
Heat
Change chapter14-1: Heat as Energy Transfer
14-3 and 14-4: Specific Heat; Calorimetry
14-5: Latent Heat
14-6 to 14-8: Conduction, Convection, Radiation
Question by Giancoli, Douglas C., Physics: Principles with Applications, 7th Ed., ©2014, Reprinted by permission of Pearson Education Inc., New York.
Problem 2
Q
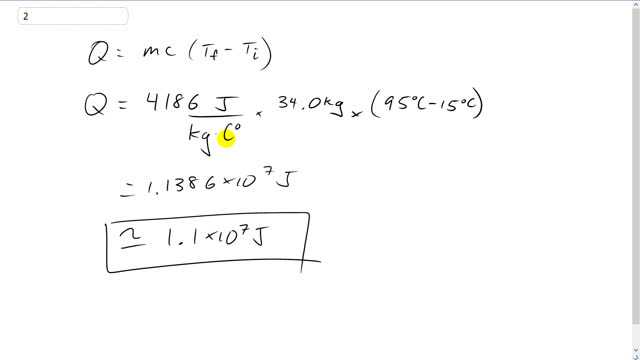
How much heat (in joules) is required to raise the temperature of 34.0 kg of water from to ?
A
In order to watch this solution you need to have a subscription.
VIDEO TRANSCRIPT
This is Giancoli Answers with Mr. Dychko. The heat gained by a substance is its mass times the specific heat capacity times the final temperature minus the initial temperature. So, that's 4,186 joules per kilogram Celsius degree for water specific heat capacity times its mass of 34 kilograms times the final temperature of 95 degrees Celsius minus the initial temperature of 15. And that gives about 1.1 times 10 to the 7 joules required to raise this temperature that much.
Giancoli Answers, including solutions and videos, is copyright © 2009-2025 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.