A 31.5-g glass thermometer reads before it is placed in 135 mL of water. When the water and thermometer come to equilibrium, the thermometer reads . What was the original temperature of the water? Ignore the mass of fluid inside the glass thermometer.
In order to watch this solution you need to have a subscription.
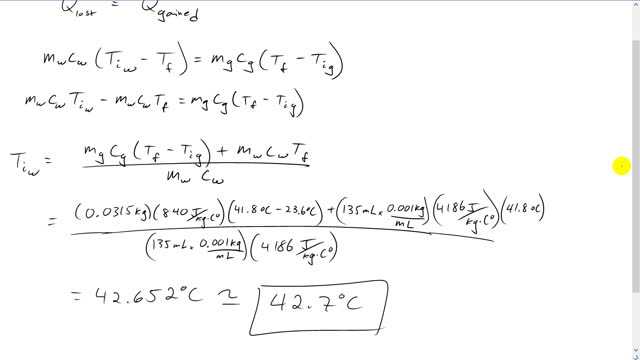
This is Giancoli Answers with Mr. Dychko. So, this question is going to show us that the act of measurement is going to change the thing being measured. So, by putting this glass thermometer in the water, we're going to be changing the water's temperature and... Well, we wanted to know what the water temperature was. And so we have to do all this work here to figure out what the temperature was given this final reading of 41.8 degrees on the thermometer. So, the thermometer reads 41.8 but that's not the temperature of the water initially before put this thermometer in. Temperature initially was 42.7. So, the heat lost by the water is going to be the heat gained by the glass thermometer. So, heat gained is final temperature minus the initial temperature and we have a subscript g for class here, times the mass, and then times specific heat capacity of glass. And the heat lost by the water is going to be the water's initial temperature minus the final temperature. Final temperature is the same for both substances. So, we don't need a subscript there for the final temperature. And we have the mass of the water times specific heat capacity of water here. So, multiplying through by mw cw we get mw cw times Tiw minus mw cw Tf. And that equals mg cg times Tf minus Tig. And then we're solving for the initial temperature of the water. So, we move this term to the right hand side which makes it plus there. And then divide both sides by mass of water times specific heat capacity of water. And we get initial temperature of the water is mass of the glass times it specific heat capacity times the change in temperature for the glass plus mw cw Tf over mw cw. So, plugging in numbers, we have mass of the glass of 0.0315 kilograms and times 840 joules per kilogram Celsius degree times its change in temperature, final temperature of 41.8 degrees Celsius, minus the initial temperature of the thermometer, 23.6 degree Celsius, plus the mass of the water which is 135 milliliters times 0.001 kilograms per milliliter times 4,186 joules per kilogram Celsius degree times the final temperature of the water, 41.8 degrees Celsius divided by the mass of the water times its specific heat capacity. And this gives 42.7 degrees Celsius must have been the temperature of the water before the thermometer was placed inside it.
