
How much heat is needed to melt 23.50 kg of silver that is initially at ?
In order to watch this solution you need to have a subscription.
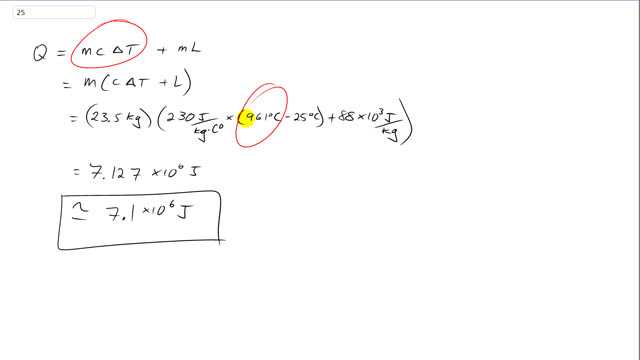
This is Giancoli Answers with Mr. Dychko. The total energy needed to melt the silver is going to be the energy needed to raise its temperature from its initial temperature of 25 degree Celsius up to its melting point of 961 degree Celsius. And then also the amount of energy needed to change its phase from solid into liquid. So, this is its mass times its latent heat of fusion. The mass we can factor out, and so we have m times specific heat times change in temperature plus the latent heat of fusion, L. So, that's 23.5 kilograms times 230 joules per kilogram Celsius degree, specific heat of silver, times the change in temperature which is this final temperature, it's a melting point of 961 degrees Celsius minus its initial temperature, 25, plus 88 times 10 to the 3 joules per kilogram, latent heat of fusion. And this gives 7.1 times 10 to the 6 joules of energy needed.