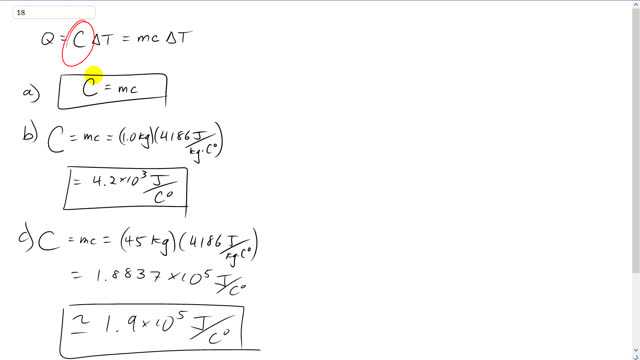
The heat capacity, C, of an object is defined as the amount of heat needed to raise its temperature by . Thus, to raise the temperature by requires heat given by .
- Write the heat capacity in terms of the specific heat, , of the material.
- What is the heat capacity of 1.0 kg of water?
- Of 45 kg of water?
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The heat gained is going to equal the heat capacity times change in temperature. So, this heat capacity is unique to each object, I mean, it's unique to each particular mass of object. So, it's the amount of energy needed to heat a specific object up 1 Celsius degree. And whereas this specific heat, little c, is the energy needed to heat up, heat something up by 1 Celsius degree per kilogram of that substance. So, the units of specific heat are joules per kilogram Celsius degree. Whereas the units for heat capacity is joules per Celsius degree. And we can convert between the two by dividing both sides of this by change in temperature. And we see that heat capacity is mass times the specific heat. So, the heat capacity of 1 kilogram of water is gonna be 1 kilogram times the specific heat of water, 4,186 joules per kilogram Celsius degree. And we get 4.2 times 10 to the 3 joules per Celsius degree. And then the heat capacity for 45 kilograms of water is 45 times 4,186 which is 1.9 times 10 to the 5 joules per Celsius degree.
