A 28-g ice cube at its melting point is dropped into an insulated container of liquid nitrogen. How much nitrogen evaporates if it is at its boiling point of 77 K and has a latent heat of vaporization of 200 kJ/kg? Assume for simplicity that the specific heat of ice is a constant and is equal to its value near its melting point.
In order to watch this solution you need to have a subscription.
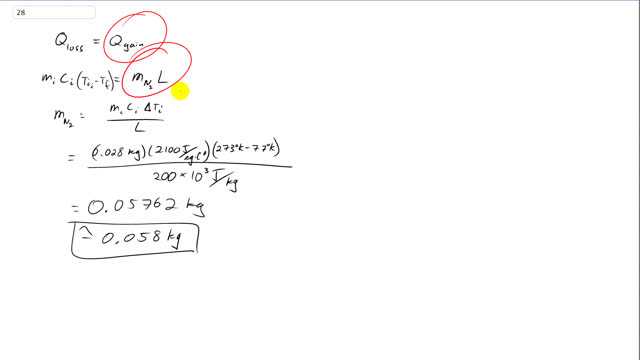
This is Giancoli Answers with Mr. Dychko. So, this ice cube is gonna get dropped into an insulated container containing liquid nitrogen. And we assume that all the energy gained by the liquid nitrogen just goes into changing its phase from liquid into gas. And we assume that the gaseous nitrogen is not affected by the ice cube. And so there's no temperature change happening there. And that makes sense because the ice cube will be submerged within the liquid nitrogen, and the gas will be, you know, above that. So, it won't be in contact with the ice cube. So, this is the amount of heat energy gained by the liquid nitrogen in order to change phase from liquid to gas. And this is the energy lost by the ice cube as it changes its temperature. And we can isolate mass of nitrogen that'll vaporize by dividing both sides by L, and switch the sides around. And so the mass of nitrogen is going to be the mass of the ice which is 0.028 kilograms multiplied by the specific heat of ice which is 2,100 joules per kilogram Celsius degree times the initial temperature of the ice minus its final temperature. And that's 273 degrees kelvin minus 77 kelvin. I guess you don't normally say degrees kelvin, you say kelvin. But, you know, it's a small point. And also it's not really good to say Δt there either because this is, you know, when you have a Δ there, it's usually final minus initial, but in this case its initial temperature of the ice minus its final temperature. OK. So, when we divide all that by the latent heat of vaporization for nitrogen, 200 times 10 to the 3 joules per kilogram. And that's 0.058 kilograms of nitrogen will change phase from liquid into gas.
