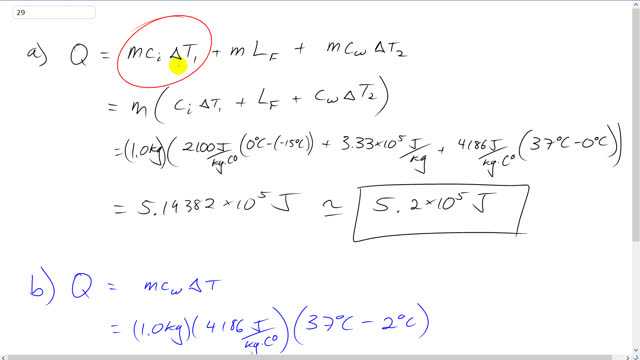
High-altitude mountain climbers do not eat snow, but always melt it first with a stove. To see why, calculate the energy absorbed from your body if you:
- eat 1.0 kg of snow which your body warms to body temperature of ;
- melt 1.0 kg of snow using a stove and drink the resulting 1.0 kg of water at , which your body has to warm to .
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The amount of heat needed to heat up some snow up to body temperature liquid water is going to be the heat absorbed to change the snow's temperature from whatever it starts at minus 15 degrees up to 0 degrees. And then after it's at 0 degrees then takes some energy to melt the snow and change its phase from solid into liquid. And then after that you have to take that liquid at 0 degrees and heat it up to 37 degrees Celsius body temperature. So, these are each of the terms corresponding to that. So, we can factor out the mass from each of the terms. And we have mass times the specific heat of ice times the ice's change in temperature plus the latent heat of fusion plus the specific heat of water times the water's change in temperature. So, we have 1 kilogram times 2,100 joules per kilogram Celsius degree, specific heat of ice, times its change in temperature which is the final temperature of 0, minus initial temperature of negative 15 and plus 3.33 times 10 to the 5 joules per kilogram, latent heat of fusion, plus 4,186 joules per kilogram Celsius degree, specific heat of water, times 37 degrees Celsius, final temperature, minus initial temperature of 0. And that gives 5.2 times 10 to the 5 joules of energy. And all that energy is going to come from the body. And in case b, where the snow has already been melted until it's water at 2 degree Celsius, the amount of energy absorbed from the body would be just the energy needed to heat it from 2 degrees to 37. So, that's 1 kilogram times 4,186 joules per kilogram Celsius degree, specific heat of water, times 37 degrees Celsius, final temperature, minus 2. And that gives 1.5 times 10 to the 5 joules which is about a quarter of the energy as it was in the first case.
