A cube of ice is taken from the freezer at and placed in an 85-g aluminum calorimeter filled with 310 g of water at room temperature of . The final situation is all water at . What was the mass of the ice cube?
In order to watch this solution you need to have a subscription.
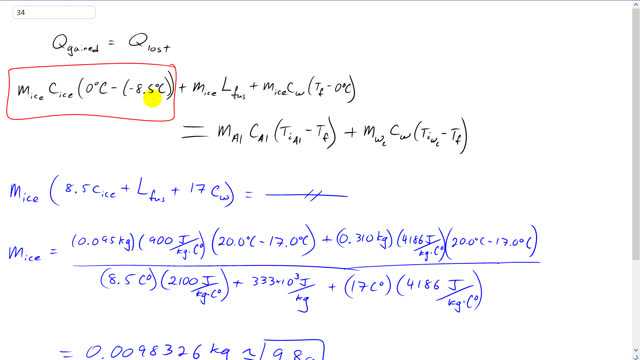
This is Giancoli Answers with Mr. Dychko. When ice is placed within this calorimeter, it's going to gain some heat to increase its temperature from its initial temperature of negative 8.5 degrees Celsius up to 0. And then once it gets to 0, it's gonna absorb some more heat energy in order to change its phase from solid into the liquid, and so that's gonna be mass of the ice times the latent heat of fusion. And then furthermore, it'll increase its temperature once it becomes water. And it'll absorb this much energy mass times specific heat of water times the final temperature minus the initial temperature of 0 which is what it has after it's done its phase change from ice into water. And I've got m ice here. Even though it's water in this term, it's the same mass as it is here and here. It's the original mass of the ice cube. And all that heat energy gained by this ice cube, which turns into water, is going to equal the heat energy lost by the aluminum that makes up the calorimeter container and the heat energy lost by the water within the calorimeter. And we can factor out m ice from all 3 terms here. And so we have m ice times 8.5 times the specific heat of ice plus the latent heat of fusion plus 17 times the specific heat of water because it ends up with a temperature of a, a final temperature of 17 degrees. So, 17 minus 0 is 17 there. And the right hand side is unchanged. And then divide both sides by this bracket. And you end up mass of the ice must be the 0.095 kilograms of aluminum that makes up the calorimeter times 900 joules per kilogram Celsius degree, specific heat of aluminum, times the initial temperature of the aluminum of 20 degrees Celsius minus the final temperature of 17 plus the 0.31 kilograms of water within the calorimeter times 4,186 joules per kilogram Celsius degree, specific heat of water, times 20 minus 17, it has the same temperature change as the aluminum did. And divide that by this whole bracket here. So, that's 8.5 times 2,100 which is the specific heat of ice plus the latent heat of fusion for water, 333 times 10 to the 3 joules per kilogram, and then plus 17 Celsius degrees times 4,186 joules per kilogram Celsius degree. And that's when the ice is turned into water and it's heating up to its final temperature 17, starting from 0. And this works out to 9.8 grams must have been the mass of the ice cube.
I believe the calorimeter is 0.085kg, not 0.095kg
Hi mattewgoffena, you have a sharp eye! Thanks for spotting that, and yes the calorimeter is 0.085kg, not 0.095kg. It turns out that the answer is 9.7686g vs 9.8326g, so to two significant figures the answer is 9.8g either way, which is why I didn't notice the error when comparing my answer with the instructor solution manual. I'll put a note above this video, and thanks again.
Best,
Mr. Dychko
