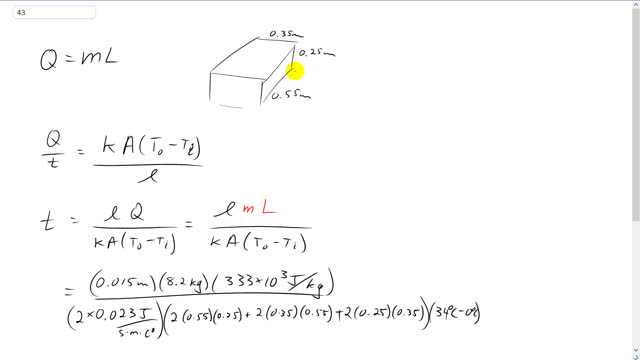
Approximately how long should it take 8.2 kg of ice at to melt when it is placed in a carefully sealed Styrofoam ice chest of dimensions whose walls are 1.5 cm thick? Assume that the conductivity of Styrofoam is double that of air and that the outside temperature is .
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. So, we assumed that when this ice is stuffed into this cooler made of styrofoam, that it's then carefully we're told. So, we can assume that there's no air spaces inside and that the styrofoam is touching the ice on all sides. And so the rate of heat transfer out of the cooler is going to be the thermal conductivity of styrofoam times the total cross sectional area of all 6 sides here times the temperature difference between the inside and outside of the cooler divided by the thickness of the styrofoam walls. And Q is going to be equal to the mass of the ice block times the latent heat of fusion because we want to know how long it's going to take for this ice to melt. So, this is the total amount of energy that has to disappear through the styrofoam walls. And so we substitute for Q as mL here. But let's solve this for t first of all. Well, probably the best way to explain it is to take the reciprocal of both sides and that makes this get flipped over, and then this also gets flipped over resulting in t over Q and then multiply both sides by Q. So, you'll see what we have here is t equals this thing flipped over times by Q. So, l times Q over kA times difference in temperatures. And Q is mass times latent heat of fusion for ice and water. So, we have the thickness of the styrofoam, 1.5 centimeters. So, that's 0.015 meters. And multiply that by the mass of the ice which is 8.2 kilograms times by the latent heat of fusion, 333 times 10 to the 3 joules per kilogram, and divide that by the thermal conductivity of styrofoam, and we're told to assume that it's 2 times the thermal conductivity of air. So, that's 2 times 0.023 joules per second meter Celsius degree, and then times it by the total area. So, there are 2 sides or 2 surfaces with area 55 centimeters by 25 centimeters. So, that's 0.55 meters by 0.25 meters. And so that's, I've shaded one side like that, and the other side you can't see it, it's on the other, behind it here. And then we have 2 surfaces that are 0.35 by 0.55. So, that's this top surface here and on the bottom and then there's two surfaces on the ends. Here's one surface of 0.35 by 0.25, and then is another one the other end. And then times that by the temperature difference, 34 degrees outside minus the temperature inside which is 0 degrees. And that gives this many seconds and we times it by 1 hour for every 3,600 seconds for a total of 8.7 hours for the ice to melt.
