If of energy is supplied to a container of liquid oxygen at , how much oxygen can evaporate?
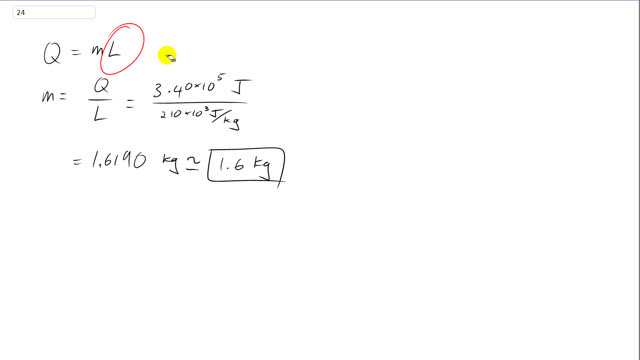
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The amount of heat needed to change phase of a certain mass of the substance is the mass times the latent heat of vaporization in this case because it's going from a liquid to a gas. So, we'll divide both sides by L to solve for m. So, the amount of mass that can turn into gas is going to be the energy absorbed which is 3.4 times 10 to the 5 joules divided by the latent heat of vaporization for oxygen which is 210 times 10 to the 3 joules per kilogram which is about 1.6 kilograms.
How did you get 2.1x10^5 j/kg? That number is not in the problem.
This number is found in a data table 14-3 titled "Latent Heats" on page 397. It's the latent heat of vaporization for oxygen.
