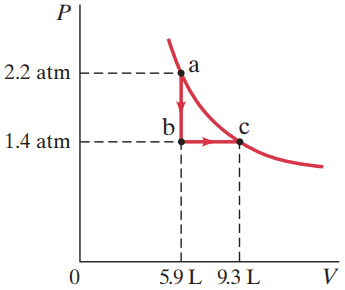
Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9 L to 9.3 L, where the temperature reaches its original value. See Fig.15–22. Calculate
- the total work done by the gas in the process,
- the change in internal energy of the gas in the process, and
- the total heat flow into or out of the gas.
In order to watch this solution you need to have a subscription.
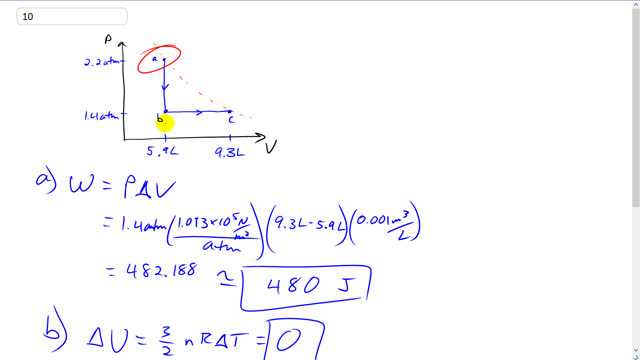
This is Giancoli Answers with Mr. Dychko. So, the gas starts at 5.9 liters with the pressure of 2.2 atmospheres and then there's an iso-volumetric transition from point A to point B during which the pressure changes from 2.2 to 1.4 but no work is done during that transition because work is going to be pressure times change in volume since there's no volume change here there's no distance over which any force is acting. So there's no work being done. And however going from point B to point C with the constant pressure and there is a change in volume you can think of work done is either the area under the graph or as pressure times change in volume, which ends up being the same thing here. Because the area under this graph here is going to be the area this rectangle of height 1.4 atmospheres and width 9.3 minus 5.9. So, we have a pressure of 1.4 atmospheres times 1.013 times ten to the five newtons per square meter for every atmosphere and that's times the change in volume, 9.3 liters minus 5.9 litres times 0.001 cubic meters per litre. And this gives 480 joules of work done by the gas. Now a change in internal energy is three ever two times n R Delta T. So three over two times number of moles times Ideal Gas constant times change in temperature but it reaches its original temperature we're told. That means there's no change in temperature between point A and point C and that makes the change in internal energy zero and the change in internal energy can also be written as the heat absorbed by the gas minus the work done by the gas. And since we know that that equals zero that makes Q equal to W and so W is positive 480 joules and so that's where Q is now and being positive means that this is absorbed by the gas. So there is heat flow of 480 joules into the gas.
