If of water at is frozen and cooled to by being in contact with a great deal of ice at , estimate the total change in entropy of the process.
In order to watch this solution you need to have a subscription.
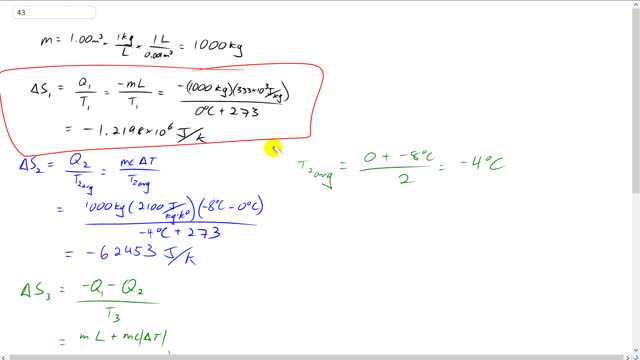
This is Giancoli answers with Mr. Dychko. There are three entropy changes to consider here. One is the entropy change resulting from the liquid water turning into ice all at zero degrees Celsius. And the amount of heat that the water will absorb is equal to mass times latent heat of fusion. But that is actually a loss in heat. So we put a negative sign there. It's you… It's this, this heat is going into the environment around it which actually is this really big block of ice which we're going to calculate its entropy change here. But first take the small one cubic meter sample of water first. And so it's going to have an entropy change due to its freezing and then it will have entropy change due to changing its temperature from, from zero degrees to minus 8 degrees Celsius and the amount of heat that it, that it absorbs is going to be <>m<>> <>c<>> <>Delta T<>> here. Mass times specific heat times change in temperature which is also going to work out to be negative as it should. Because it's giving off all this heat as it gets cooler and cooler and the negative will come from this <>Delta T<>> here, negative eight degrees Celsius minus zero is going to get negative eight. So all of this, all of the heat that the one cubic meter sample of water has released is going to be absorbed by the ice that it's been placed into. And so when we calculate the entropy change of the ices surrounding it. It's going to absorb the amount of heat due to the water freezing and it's going to absorb some heat due to the water cooling down to minus eight degrees and all this is going to happen at minus eight degrees Celsius. And the clue that this temperature is constant is that, is that we're told that this… that this thing is in contact with a great deal of ice. So because it's a great deal of ice that means it's so massive that it its temperature is not going to significantly change when this heat is added to it. So when then we'll add all these entropy changes together to get our final result for the total entropy change in the process. so entropy change of the one cubic meter of water as a result just of its phased in transition. Well one cubic meter is multiplied by density of one kilogram per liter. And then times by one liter for every point 0.001 cubic meters we get 1000 kilograms is the amount of water we're dealing with. And so the change in entropy as you change from liquid into solid is a mass times latent heat of fusion divided by the temperature. And this is all happening at zero degrees Celsius. So we have a 1000 kilograms times 333 times ten to the three joules per kilogram. This is negative because its losing this much heat to its surrounding and divided by zero degrees Celsius expressed in Kelvin by adding 273 and we get negative 1.2198 times ten to the six joules per Kelvin. Its reducing its entropy and… and then its going to lose some more heat due to its change in temperature. So we have a 1000 kilograms times the specific heat of ice which is 2100 joules per kilogram Celsius degree or Kelvin whatever you like to say, times negative eight degrees Celsius, final temperature, minus three degrees Celsius initial temperature and all that's divided by the average temperature and the average temperature is the average of zero and negative eight which is negative four and we write that in Kelvin by adding 273 and we get negative 62453 joules per Calvin, change in entropy there. And then a change in entropy of the great deal of ice that's surrounding the sample of water which turns into ice is gonna be the total heat added to it which is the total heat lost by the water divided by the temperature of the great deal of ice. So we have the heat due to the phase transition mass times latent heat of fusion plus the heat gained by the surrounding ice equal to mass times specific heat times the change in temperature and that's factor at the end when we have a 1000 kilograms times 333 times ten to the three joules per kilogram plus 2100 joules per kilogram kelvin times eight Celsius degrees and we have and this is all happening at negative eight degrees Celsius, so add 273 to that’ll convert into Kelvin and the change in entropy of the surrounding ice is 1.32 times ten to the six Joules per Kelvin. Add this and this and then add this number and all those together makes 3.8 times ten to the four Joules per Kelvin which is the overall change in entropy of the system and we expected this to be positive as it is for any natural process and this is a natural process where you can take some water put it in the big block of ice and then the water will freeze and. And as for any natural process the change in entropy is always positive.
