Problem 18
Q
A heat engine exhausts 8200 J of heat while performing 2600 J of useful work. What is the efficiency of this engine?
A
In order to watch this solution you need to have a subscription.
VIDEO TRANSCRIPT
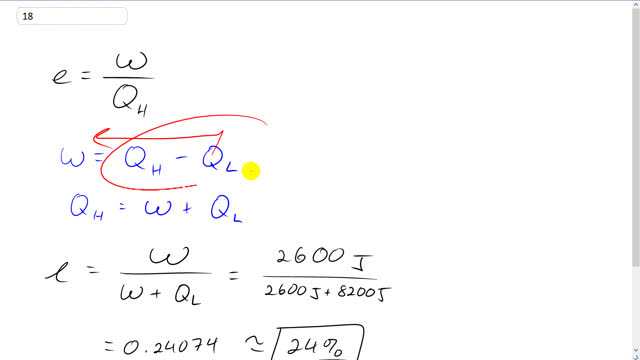
This is Giancoli Answers with Mister Dychko. Efficiency is the work done by the engine divided by the amount of heat added to it from the high temperature reservoir. Work done is also the difference between the heat added minus the heat lost of the low temperature reservoir. We can move this over to the left side and then switch the signs around and get Qh is W plus QL. And then we'll substitute that in for Qh up here. So, W plus QL, doing my substitution in red. So, that's 2600 joules of work done divided by 2600 plus 2800 joules. This is an efficiency of 24 percent.
Giancoli Answers, including solutions and videos, is copyright © 2009-2026 Shaun Dychko, Vancouver, BC, Canada. Giancoli Answers is not affiliated with the textbook publisher. Book covers, titles, and author names appear for reference purposes only and are the property of their respective owners. Giancoli Answers is your best source for the 7th and 6th edition Giancoli physics solutions.
