A 2.8-kg piece of aluminum at is placed in 1.0 kg of water in a Styrofoam container at room temperature . Estimate the net change in entropy of the system.
In order to watch this solution you need to have a subscription.
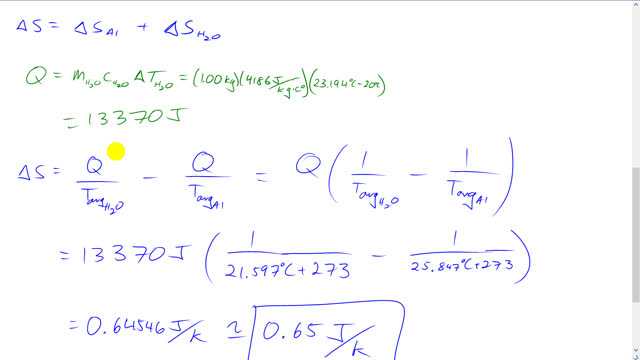
This is Giancoli answers with Mr. Dychko. In order to calculate changes in entropy we need to know what the average temperature is of each of these substances the aluminum in the water, coz we are Going to take the heat gained by the water and divide by the water's average temperature and then take the heat lost by the aluminum and divide by the aluminum’s average temperature. But to know what these average temperatures are we need to know what the final equilibrium temperature will be of the aluminum and water after they put together. And so we calculate that here for the… we calculate the averages here by taking the final plus the initial and divided by two and points to know what this final temperature is by using this calorimetry stuff that we learned in the last chapter. So we have the the Heat lost by the aluminum equals the heat gained by the water and that means we have temperature final minus initial for the water and on the lost side we have initial minus final temperature for the aluminum. So this is mass of aluminum times aluminium’s specific heat times the change in temperature initial minus final. And then that equals the mass of water times water’s specific heat times the final temperature minus the water's initial temperature. No need for a subscript on the final temperature to distinguish water from aluminum because they both will reach the same final temperature. So distribute this m c into the brackets on both sides and then collect the terms that contain T f on the same side. So this comes to the right side becoming positive and this comes on the left side becoming positive as well. And so this is the entire left side and… but switch the sides around as well, after moving them and then factor out the T f and divide by this m ai c ai plus m h2o c h2o and to get this line after and… and then plug in some numbers. So 2.8 kilograms of aluminum times 900 joules per kilogram Celsius degree, specific heat of aluminium, times its initial temperature of 28 and a half degree Celsius. And this doesn't have to be in Kelvin because when you're taking a difference like this a difference in Kelvin is the same as a difference in Celsius because they have the same size units. Not true for Fahrenheit and but… and then add one kilogram of water times 4186 joules per kilogram Celsius degree there, times its initial temperature of 20 degrees Celsius and then divide by the mass of aluminum times its specific heat plus the mass of water times its specific heat. This gives 23.194 degrees Celsius as the final temperature. So it's a long question because in the last chapter this would have been the end of our question and just find the final equilibrium temperature. But that's just part of what we do here. So here we need to find the average temperature the aluminum and so that's the initial temperature of 28 and a half plus his final temperature 23.194 and divide by two and it gives 25.847 degrees Celsius. The average temperature for water is 20 degrees Celsius plus the final divided by two which is 21.597 degrees Celsius and the total change in entropy will be the change in entropy of the aluminum plus the change in entropy of the water. So in order to calculate that we also need to know what is the heat gained or lost by the substances. So we figured out the average temperature is now and then let's figure out the heat transfer. So we'll consider the heat gained by the water which is going to be of equal magnitude to the Heat lost by the aluminum because that's where the heat came from in the first place. So we have mass of water is one kilogram times its specific heat of 4186 joules per kilogram Celsius degree times the change in temperature which is the final temperature minus the initial and we get 13370 joules. and so there's no need for a subscript on the Q to distinguish them between these water and the aluminum terms because they have equal magnitudes of the heat transfer is whatever is lost but aluminum is the same amount gain by the… the water but we have this minus here to say that the aluminum has lost this heat there. So factor out the Q and so we have Q times one over the water’s average temperature minus one over the aluminum’s average temperature and that makes 13370 Joules times one over the temperature of water in Kelvin, 21.597 degrees plus 273 minus one over the average temperature of aluminum in Kelvin and that gives 0.65 joules per Kelvin is the change in entropy of the system and we expected a positive number here because this is a natural process and and as for any natural process the total change in entropy should be positive.
