8.5 moles of an ideal monatomic gas expand adiabatically, performing 8300 J of work in the process. What is the change in temperature of the gas during this expansion?
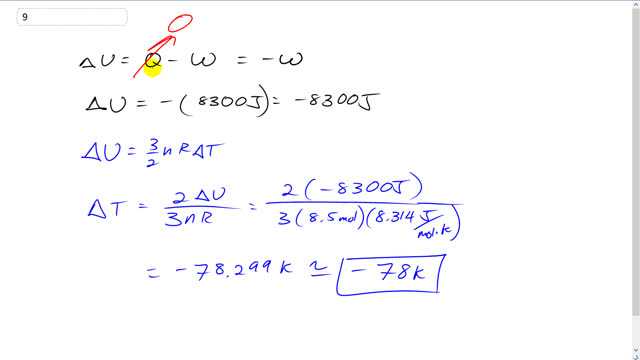
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. Change in internal energy is the heat absorbed by the gas minus the work done by the gas. But the process here we're told is adiabatic. So that means the heat absorbed is zero, there's no heat exchange at all. And so that makes internal energy change equal to the negative the work done by the gas. So the gas does 8300 joules of work and so that means change in internal energy is the negative of that work done by the gas. And so that makes the negative 8300 joules is the change in internal energy. Change in internal energy also can be thought of as three over two times the number of moles times the Ideal Gas constant times change in temperature and we can rearrange this by multiplying both sides by two over three n R to solve Delta T. So change in temperature is two times the change in internal energy over three n R. So that's two times negative 8300 joules divided by three times eight and a half moles times 8.314 joules per mole Kelvin, Ideal Gas constant which is negative 78 Kelvin, is the amount that the temperature will change, so it’ll drop 78 Kelvin.
