A heat engine exhausts its heat at and has a Carnot efficiency of 36%. What exhaust temperature would enable it to achieve a Carnot efficiency of 42%?
In order to watch this solution you need to have a subscription.
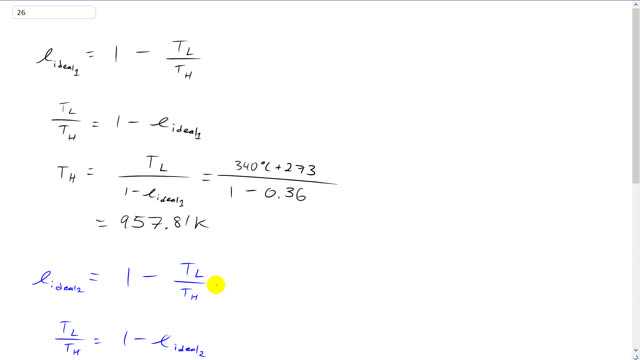
This is Giancoli Answers with Mr. Dychko. The first thing we need to do is figure out what is the high temperature that we're working with and then we'll use the first efficiency to calculate what that is and then consider the second efficiency and figure out what low temperature would work with that second efficiency and the high temperature that we figured out from the first part. So let's go through that step by step. So the first efficiency is one minus the low temperature divided by the high temperature and we can move this to the left side making a positive and move it to the right side making it minus and low temperature divided by high temperature is one minus the efficiency ideal efficiency in the first case and that makes the well I guess how to explain how to get the TH there. Let's say take the reciprocal of both sides. In which case this becomes one over one minus the ideal one. And on the left side you have TH over TL the multiply both sides by TL and you get this line here. So the high temperatures low temperature divided by one minus ideal efficiency in the first case and that's 340 degrees Celsius converted into Kelvin by adding 273 and divide that by one minus 0.36 which gives 957.81 Kelvin is the high temperature. Now we're going to change the low temperature such that we have an efficiency of 0.42 instead of 0.36 and the high temperature is gonna stay the same as it was before. And that same process here move this to the left move this to the right and we get TL over TH equals one minus ideal efficiency two. And I guess I can call this TL two . It's a new low temperature whereas before it was TL one and then multiply both sides by TH and you get the low temperature for the second efficiency is the same high temperature is before times one minus this new ideal efficiency. So that's 957.81 Kelvin times one minus 0.42 which is 555.53 Kelvin and take away 273 to get the answer in degrees Celsius which round to 280 degrees Celsius.
