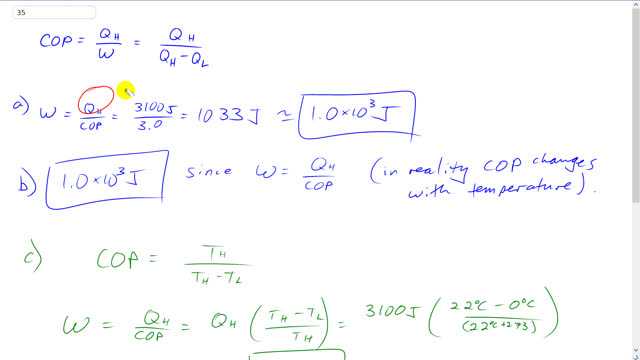
A heat pump is used to keep a house warm at . How much work is required of the pump to deliver 3100 J of heat into the house if the outdoor temperature is
- ,
- ? Assume a COP of 3.0.
- Redo for both temperatures, assuming an ideal (Carnot) coefficient of performance .
In order to watch this solution you need to have a subscription.
This is Giancoli answers with Mr. Dychko. The work done by this heat pump is the amount of heat delivered to the high temperature region which is its job it has to deliver some heat into the house on a cold day and divided by the coefficient of performance of the heat pump. So it's 3100 joules of heat delivered divided by three which gives 1.0 times ten to the three joules of work done to deliver this much heat and we expected the work done to be smaller than the amount of heat delivered because that's the benefit of a heat pump. You put in some work and then that work gets delivered as heat. But then in addition you're pulling some heat energy from a low temperature reservoir as well. And so this total heat delivered should be more than the work done to deliver it. In part B it's the same answer. It doesn't matter what the temperature is outside although in reality the coefficient of performance will change with temperature. The only answer we can give for Part B is still 1.0 times ten to the three joules because we don't know in what way the coefficient of performance would change when the temperature is minus 15 degrees Celsius outside. So we just have to assume it's still three in which case the calculation is the same. And for Part C, assuming an ideal coefficient of performance, the work done is going to be the heat delivered divided by this coefficient performance which is the same as multiplying by the reciprocal of it. So we'll take Q H multiplied by T H minus T L divided by T H. So that's 3100 joules times 22 degrees Celsius minus zero outside, divided by 22 degrees Celsius plus 273 to convert it into Kelvin. We could have converted both the temperatures in the numerator here into Kelvin by adding 273 but it's going to be plus 273 and then minus plus 273. So that just cancels out anyway. So, we have 230 joules of work done when the temperature outside is zero. When the temperature outside is minus 15, it's going to be 22 degrees Celsius minus negative 15 on top and the denominator is the same. Still multiply by 3100 joules same amount of heat delivered but it's going to take 390 joules of work in this case and that makes sense that it takes more work to deliver the same heat when the temperature outside is lower. And there we go.
you did not do a with 0 degrees celsius
