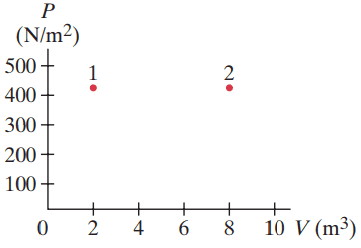
The PV diagram in Fig. 15–23 shows two possible states of a system containing 1.75 moles of a monatomic ideal gas. , ,
- Draw the process which depicts an isobaric expansion from state 1 to state 2, and label this process A.
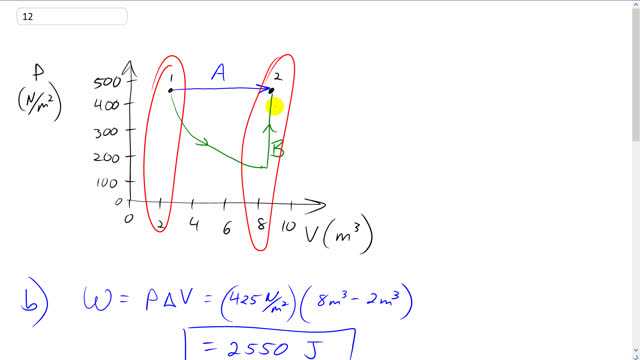
- Find the work done by the gas and the change in internal energy of the gas in process A.
- Draw the two-step process which depicts an isothermal expansion from state 1 to the volume , followed by an isovolumetric increase in temperature to state 2, and label this process B.
- Find the change in internal energy of the gas for the two-step process B.
- see video
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. So, for part A we have an isobaric expansion from this initial volume of two cubic meters to a final volume of eight cubic meters with a pressure of 425. And that's process A labeled in blue. Part B says find the work done by the gas and the change in internal energy during this process A. So the work done is the constant pressure times a change in volume. So it's 425 newtons per square meter times eight cubic metres final volume minus two cubic meters initial volume which is 2550 joules of work done by the gas. And the change in internal energy is three over two times number of moles times R times change in temperature And we know that temperature two is gonna be PV2 over nR. If we're rearranging this PV equals nRT formula, we can solve this for T and say that T is PV over nR. So temperature two, the temperature here is going to be whatever that pressure is times volume two divided by nR and temperature one is gonna be the same pressure, because it's isobaric, times its V1 divided by nR and that means change in temperature is T2 minus T1 which is P V two over n R minus P V one over n R. And we can factor out the P over nR and that's going to be multiplied by V2 minus V1 which is change in volume and substitute that in for Delta T in this Delta U formula. So we have three over two nR times P Delta over nR and the nR cancels, leaving us with three over two P Delta V. So the change in internal energy is, three over two times the work done by the gas as it turns out. So it's three or two times 425 newtons per square meter times eight cubic metres minus two which is 3.83 times ten to the three joules change in internal energy. Now change in internal energy depends only on change in temperature because of this formula. And we can see that the change in temperature is the same for both these processes because they both started the same temperature because process B goes first of all along this isotherm which means same temperature and so they start at the same temperature here and they end at the same point which is on other isotherm. And so they transition between the same two isotherms so that they have the same change in temperature for both these processes and that means that they have the same change in energy. So change in energy for process B is the same as that for A and so that's also 3.83 times ten to the three Joules.
