An aluminum rod conducts 8.40 cal/s from a heat source maintained at to a large body of water at . Calculate the rate at which entropy increases in this process.
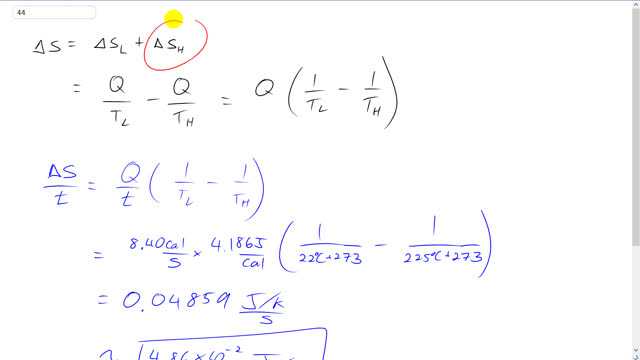
In order to watch this solution you need to have a subscription.
This is Giancoli answers with Mr. Dychko. The total entropy change is the change in entropy of the low temperature Reservoir which is the body of water plus the change in entropy of the high temperature thing which is the heat source. And so they're both going to have the same transfer of, of heat but in the case of the low temperature reservoir is gonna be gaining that heat to have a positive Q whereas the heat source is going to be losing this amount of heat. And so we have a minus Q there and divided by T L for the low temperature thing and then divided by T H high temperature in the case of the heat source and we can factor out this Q and we get Q times one over T L minus one over T H is the total change in entropy of the system and we can divide both sides by time if you like and in this case we're dividing by one second. So we have 8.4 calories per second and then times that by 4.186 joules per calorie, you get to her regular SI units and then times by one over 22 degrees Celsius converted into Kelvin by adding 273 minus one over the temperature of the heat source in Kelvin and we get 4.86 times ten to the minus two joules per Kelvin per second. And you can write this as this if you like, both mean the same thing and there we go.
