One liter of air is cooled at constant pressure until its volume is halved, and then it is allowed to expand isothermally back to its original volume. Draw the process on a PV diagram.
In order to watch this solution you need to have a subscription.
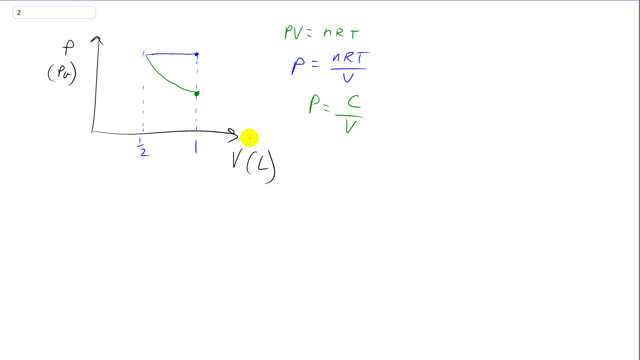
This is Giancoli Answers with Mr. Dychko. Here's the ideal gas law. PV equals nRT and we can use this to help us figure out what this picture should look like on the P versus V diagram pressure versus volume. So we started some initial volume, we'll call it one and then we go to at constant pressure. We go to a volume of one half what it was before. And we can see that what's happening here is that the if the volume is gonna be decreasing, then the temperature must also be decreasing in the same proportion in order for P to remain constant during this blue section here. So if volume is going to decrease by a half, then T also has to decrease by half in order for this ratio to stay the same. Because since pressure is constant, that means this whole nRT over V has to be constant while the volume is changing. And that's only possible if T changes in the same proportion or changes by the same factor, that the other way to say that. So that explains the blue section. So temperature is decreasing as the volume is decreasing and the pressure is constant and then the return trip back to the original volume of one is going to follow a different path when the transition is Isothermic, because now we're going to be holding temperature constant as we increase the volume and so that makes this numerator nRT a constant. Let's just call it C. So I've written this equation in green to say that it describes this section here going from the small half volume back up to this volume of one along this Isothermic transition and so we have just a constant up here nRT everything's constant and the number of moles it's going to be the same as a sealed container times the ideal gas, guns constant times, this constant temperature and during this transition in green. And so it's just some constant C divided by V. So if we have a constant divided by this V and this V is increasing, as V increases, the fraction gets smaller. So if you have one quarter as this, that's smaller than one half. So as you increase the denominator, you end up with a smaller quotient. And so pressure is decreasing as you get to a larger volume when you hold temperature constant. And so that explains this picture here. This is the reciprocal graph that resembles the you know, if you have y equals one over x, that's what this picture is here. So, you know, it's not one and some constant C, but it's the same idea, same shape.
