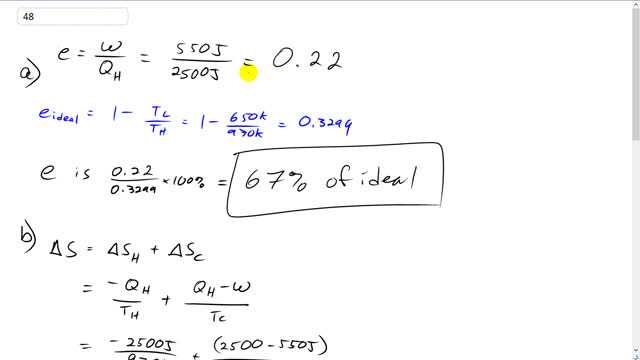
A real heat engine working between heat reservoirs at 970 K and 650 K produces 550 J of work per cycle for a heat input of 2500 J.
- Compare the efficiency of this real engine to that of an ideal (Carnot) engine.
- Calculate the total entropy change of the universe per cycle of the real engine, and
- also if the engine is ideal (Carnot).
- 0
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The efficiency of this heat engine is the work that it does divided by the total amount of heat that it absorbs from a high-temperature reservoir. So it's 550 Joules divided by 2,500 Joules which is .22. Its ideal efficiency is one minus the temperature or the low temperature reservoir divided by the high temperature. So that's one minus 650 Kelvin divided by 970 Kelvin, which is .3299. So the efficiency is .22 divided by .3299 times 100 percent is 67 percent of the ideal. The total change in entropy of the universe is going to be the change in entropy of the hot reservoir plus the change in entropy of the cold temperature reservoir. So the hot temperature reservoir losses a certain amount of heat, Q H, and because of losing that-- Let's probably put a negative sign in front of it. And we'll divide that by the hot temperature to get the change in entropy of the hot temperature reservoir, and then add to that the heat that's gained by the low-temperature reservoir which is the total heat absorbed from the hot temperature reservoir minus the work that is done. That together makes Q L. And that's divided by the temperature of the cold temperature reservoir. So we have negative 2,500 Joules divided by 970 Kelvin, plus 2,500 Joules absorbed minus 550 Joules work done, divided by 650 Kelvin, which is .42 Joules per Kelvin total change in entropy. Now we're going to calculate the change in entropy if the engine is an ideal engine. So, in that case, the calculation is the same as before except we're going to have different amount of work done. That's not going to be 550 Joules anymore, it's going to be the ideal amount of work done. Let's figure out what that is. So ideal efficiency is high temperature minus the cold temperature, divided by the hot temperature. And the ideal amount of work done is the ideal efficiency times the heat absorbed from the high-temperature reservoir. So we can substitute for e ideal from this here. And then multiply it through by Q H and we get the ideal amount of work done is Q H times T H minus Q H times T C, over T H. Then just focusing in just in that numerator, before we do anything substitution into there, that numerator is going to be now Q H minus W ideal, which is Q H times T H over T H in order to give it a common denominator, minus all this. And this minus sign is distributed into the brackets, and we end up with a Q H T H minus Q H T H plus Q H T C. And these two terms are the same and so they make zero with opposite signs. And so we get, in the end, positive Q H T C over T H, that's the heat absorbed minus ideal amount of work done. That is what we'll substitute in for this numerator, and then we multiply it by one over T C. So it's the same as dividing by T C is multiplying it by its reciprocal one over T C and we see that the T Cs cancel, and we're left with negative Q H over T H plus Q H over T H which is zero. And so this is the expected result when you have an ideal engine that you'll have the minimum change in entropy occurring which is zero.
