A heat engine uses a heat source at and has an ideal (Carnot) efficiency of 22%. To increase the ideal efficiency to 42%, what must be the temperature of the heat source?
In order to watch this solution you need to have a subscription.
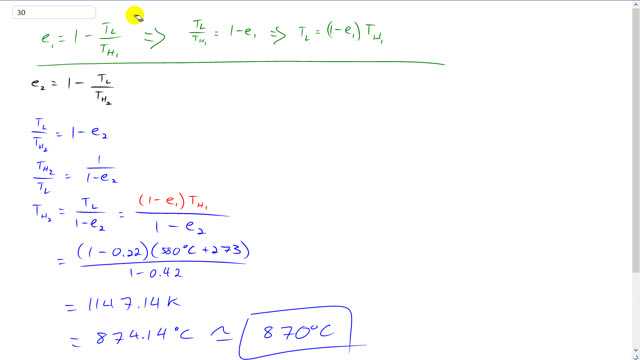
This is Giancoli Answers with Mr. Dychko. We have two different efficiencies and we have the same low temperature heat sink and we are gonna change the high temperature heat source such that the efficiency improves from 22 percent up to 42 percent. So in this expression for the second efficiency we don't know TH Two . And that's we're gonna have to find. And we also don't know TL but we can figure it out based on this first efficiency and so we'll do a bit of algebra here in green to figure out TL then substitute in for that in our second efficiency equation. So efficiency one is one minus the low temperature divided by the high temperature in the first case and then rearranging that a bit moving this to the left side and moving this to the right side gives us a positive TL over TH one equals one minus efficiency one and multiply both sides by TH One and you get the low temperature is one minus efficiency one times high temperature one and we're gonna hold onto that for a minute and then turn down our attention to the second efficiency and it's one minus low temperature divided by high temperature two and move this to the left and move the efficiency turn to the right and you get TL over TH Two equals one minus Efficiency Two then take the reciprocal of both sides because what we're trying to do is solve for TH Two . So taking the reciprocal left side flips that fraction that becomes TH Two over TL and on the right hand side there was originally one minus e One all over one and flipped that fraction and you get one over one minus efficiency two and then multiply both sides by TL and so the high temperature in the second case is gonna be the low temperature divided by one minus efficiency two and the low temperature is one minus e one times TH One and so we have one minus the 0.22 the efficiency in the first case times the high temperature in the first case which was 580 degrees Celsius then add 273 to convert it to the Kelvin and divided by one minus efficiency two that we want 0.42 and this gives 1147 Kelvin. and then take away 273 to convert it into degrees Celsius and it's about 870 degrees Celsius and then take away 273 to convert it into degrees Celsius and it's about 870 degrees Celsius that would give us an efficiency of 42 percent instead of 22 percent.
