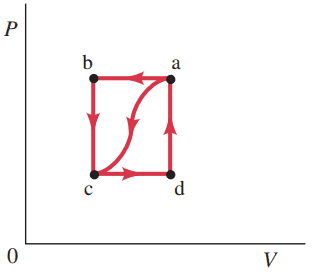
When a gas is taken from a to c along the curved path in Fig. 15–24, the work done by the gas is W = -35 J and the heat added to the gas is Q = -175 J. Along path abc, the work done by the gas is W = -56 J. (That is, 56 J of work is done on the gas.)
- What is Q for path abc?
- If , what is W for path cda?
- What is Q for path cda?
- What is ?
- If , what is Q for path da?
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. So to answer questions with this PV diagram we'll need to keep this formula in mind that the change in internal energy is the heat absorbed by the gas minus the work done by the gas. And what we're using subscript here ac and abc and so on. We're always ordering them as the starting point and then the destination. So W ac is the work done in the process from A to C and W ac means directly from A to C. So it means along this curve path. Whereas the work done abc means along the path starting at a going to b and then to c, there's different amounts of work done along those two paths because it's a different area underneath the Pv diagram. Here w ac area and here is w abc. So the work done from a to c is negative 35 Joules and the amount of heat absorbed by the gas is negative 175 Joules which means it lost lots of heat and just to to think about how this works here. So there is work done on the gas which would tend to increase the temperature of the gas. You would think anyway. Because when there is work done on the gas this w becomes negative and this minus a negative mix this Delta U positive which would tend to make Delta T positive but there's a compensatingly larger extraction of heat from the gas that's absorbing a negative amount. And so in the end this difference becomes negative because negative 175 Joules of energy lost and then 35 Joules of work done. There is negative 35 Joules of work done by the gas which is to say that there's 35 Joules of work done on the gas and anyway so this this negative of a negative makes a positive 35 and then the total change in energy though is still going to be negative because it's 175 Joules of heat absorbed negative, plus the 35 so you end up with a negative change in internal energy. So that's how you get from this point of high temperature to a point of low temperature despite work being done on the gas. There's so much more heat taken out of it. Well it doesn’t really answer any of our questions but it just helps to characterize what's happening here. And then the work done from A to B to C we're told is negative 56 Joules and we should observe that there's no work done from B to C because there's no change in volume. And so this is also the same as the work done just from A to B. First step is what is Q for path A B and C. The change in internal energy from A to C is the same as the internal energy change from A B C because they have the same endpoints and internal energy change only depends on the endpoints that depend only on the change in temperature. And starting from A going to C whatever path you take doesn't matter so far as the change in internal energy is concerned because you're going from between equal temperatures. So substituting for a Delta U ac, that's the heat absorbed by the gas minus the work done by the gas from A to C, it too equals the heat absorbed by the gas on the path ABC minus the work done by the gas along path ABC. And then we'll take this to the right hand side. Whoops sorry. We'll take this one to left hand side, I should say. Which makes it positive. And then switch the sides around to get Q abc. So Q abc is as, Q ac minus W ac plus W abc and so the heat absorbed by the gas from Q ac along this this curve here is negative 175 Joules and then minus the work done from A to C is negative 35 Joules and then plus the work done from A to B to C we're told is negative 56 Joules. So you combine all this together, being really careful with the negative sign so negative of negative make plus 35 in the end there. It works out to negative 196 Joules of heat absorbed by the gas. So this is to say 196 Joules have been extracted as another way to say that. So absorbing negative 196 is the same as emitting positive 196 and it makes sense that this number is more negative than this number because in the path ABC there's more work being done on the gas and work done on the gas would tend to increase temperature. And so there needs to be a compensating a larger extraction of heat to arrive at the same destination as you had between path along path AC. So along path AC had to extract only a 175 Joules because there is less work being done on the gas. Whereas in the path A to B to C there's more work being done on the gas and so you have to extract even more heat in order to arrive at the same temperature. So we expected a number less than negative 175, negative 196. Makes sense. So Part B if the pressure at point C is one half the pressure at point B what is the work done for the path C B A. Whoops sorry CDA. Yeah. C to D to A which is the area under the curve here. The work done from C to D to A is the same as the work done from C to D because there's no work done from D to A. Since the volume is a constant there. And this is going to be the pressure at C times change in volume from C to D. That's the work calculation when you have constant pressure is you take the pressure times by the change in volume. And the change in volume from C to D is the opposite of the change in volume from A to B. So opposite means negative of the change. Same magnitude but opposite sign. It's increasing here whereas it was decreasing from A to B. And we're told that pressure C is one half pressure B. So we substitute both of those into our work formula for CDA, pressure C is one half pressure B and the volume change from C to D is the negative of the volume change from A to B and take out the negative in the one half and you end up with this which is the work done from A to B. We know what that is. There it is. Yay! We're told the work done from A to B is negative 56 Joules. So we can substitute in negative 56 Joules here. So we have negative one half times negative 56 is positive 28 Joules and we expected a number smaller in magnitude for the work done here because this is a smaller area under this curve from C to D. And the work is positive because the gas volume is increasing. And so it means there's positive work being done by the gas since it's increasing its volume. And so we expected a positive number of smaller magnitude than we had for the work here. And that's exactly what we have. And that's good. And then what is Q for path CDA. Change in internal energy from C to D to A. That's CDA is going to be equal to the change in internal energy from A to C because we're going between equal points. So change internal energy is always the same between the same two points regardless of what path you take. But the change in internal energy will be the opposite sign because we're going in the opposite direction between those two points. So from C to D to A is going to be the opposite to change internal energy as there is from A to C. So that's what this says. And then substituting for these with the Q minus W business we have change internal energy from CDA is the heat absorbed by the gas CDA minus the work done by the gas from CDA and that equals a negative of Q ac minus W ac and bring this to the right hand side which makes it a positive. And then distribute the negative in there and you have negative Q ac and then plus W ac. And work from C to D to A, we've already calculated as 28 Joules. So we plug that in, heat absorbed from A to C we know is negative 175. So plug that in and this minus outside and then the negative value is going to end up being positive there and then plus the negative 35 Joules of work done by the gas from A to C. And this works out to 168 Joules of energy absorbed and from C to D to A and it kind of makes sense to have energy heat being absorbed since it's increasing its temperature. So there we go 168 Joules of heat absorbed and then what is U a minus U c. So U a minus U c is the same as Delta U ca, so it's starting at C ending at A. Is there anything else I need to say about that? Because have a Delta it's always a Delta something, it's always final minus initial right. So here we have... The way I've written my subscripts here it's always you put the thing that you start at and then the second subscript is the place where you end. And so to have final minus initial and they have... Basically I'm writing U initial final is the way I'm ordering the subscripts, start and then end. And so that's how this corresponds to when you write them as individual terms it's like this. So the subscript are initial final, CA, and that's going to be the final one minus the initial one and so since we're told that A is the final one that means it goes as the second subscript and C is the initial one that goes as the first subscript. And then that's gonna be the opposite of the change in internal energy from A to C. And because it's going between the same two points just in the opposite direction. So that means opposite sign for change in internal energy and that's gonna be negative of Q ac minus W ac, and Q ac and W ac are both given to us negative 175 Joules for Q ac and the negative 35 Joules for W ac. And they end up with positive 140 Joules and this makes sense because we expect this point to have a higher internal energy than this point since there is a higher temperature at A than there is at C. Your PV diagram, you can think of there being a whole bunch of isotherms along here. Each of these curves represents increasing temperature. So, temperature is increasing as you go this way to each successive isotherm. So, point A should have a higher temperature than point C and therefore a higher internal energy. And so we expected a positive answer when we go U a minus U c and there we have it, positive140 Joules. Last but not least. Knowing that U d minus U c is 42 Joules, and according to our subscript system that's going to be a Delta U cd, knowing that that's 42 Joules. What is Q for the path DA from D to A there? Well, the internal energy change from C to A going from here to here is the sum of the internal energy changes along the path, so the internal energy change from C to A is the internal energy change from C to D plus the internal energy change from D to A. And that's what this says. And the internal energy change along that vertical, that iso-volumetric part is going to be a Q da minus W da. But there's no work done since it's iso-volumetric. So that term is gone and we can solve for Q da and it's gonna be Delta U ca minus Delta U cd. So, U ca is 140 Joules as we know from Part D and U cd is… UCD. Ooh, where is that one? Oh, it's given to us. Yeah, there we go, 42 Joules. That's nice. And so our answer is 98 Joules.
These answers are not the same as they are in the back of the book.
Hi matthewgoffena, thanks for the comment. I'm away from the book at the moment, but I'll have a look at this on Saturday. I have noticed some mistakes in the answers at the back of the book, so this might be the case here, but I'll check to make sure on Sat. If you notice any specific mistake in the video, please let me know.
Hi mattewgoffena,
I took a look at this question, there don't seem to be any mistakes, so the answers in the back of the textbook are incorrect. This isn't the first time I've noticed a mistake in the back of the book. If you have any questions about the steps in the video, just let me know.
Cheers,
Mr. Dychko
