Which will improve the efficiency of a Carnot engine more: a increase in the high-temperature reservoir, or a decrease in the low-temperature reservoir? Give\ detailed results. Can you state a generalization?
In order to watch this solution you need to have a subscription.
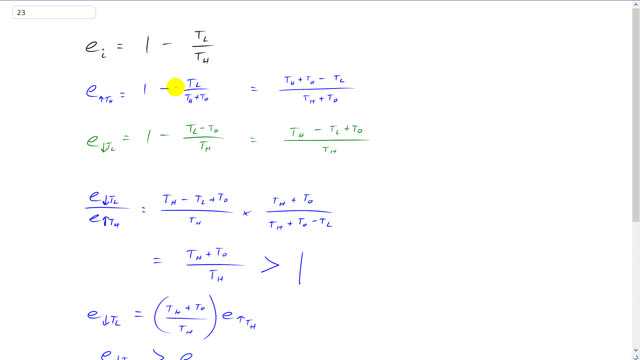
This is Giancoli Answers with Mr. Dychko. So ideal efficiency is one minus the temperature of the low temperature reservoir divided by the temperature of the high temperature reservoir. Now if you were to increase the temperature of the high temperature reservoir your efficiency would be one minus TL over TH plus whatever you decide to increase it by. So this could be ten degrees. So this is where we're adding ten degrees to the high temperature and the efficiency if you reduce the low temperature would be one minus. Low temperature minus that same amount. And we can read each of these as single fractions because we're going to do next is going to divide them and see by what factor they're different. So we'll multiply this one by TH plus To over TH plus To. We're making a common denominator here. So this becomes TH plus To minus TL all over TH plus To . And for the reduced lower temperature we're gonna multiply this one by TH over TH and this gives us one minus and there's a bracket here always implied and a fraction. That's one minus TL and then minus negative makes positive To there over TH . So let's divide these efficiencies so dividing the reduced lower temperature by the efficiency when you raise the high temperature is gonna be the low temperature efficiency TH minus TL plus To over TH just copied and then instead of dividing by this fraction we're going to multiply by its reciprocal for the raise high temperature efficiency. So that's gonna be multiplied by TH plus To over TH plus To minus TL and a handy thing is that these are the same there. I wrote the terms and different orders but they all contain the same quantities. So we have a positive TH in both of them. We have a positive To in both of them and we have a minus TL in both of them so these cancel. So the ratio of efficiencies is TH plus To over TH which will always be greater than 1. The numerator is always going to be more than the denominator here. And so we have efficiency when you reduce the lower temperature there's gonna be some number greater than one multiplied by the efficiency when you raise the high temperature and that means the reducing the low temperature efficiency is always going to be greater than the efficiency when you raise the high temperature. And this is true for ten degrees Celsius degrees like they suggest or any amount that you decide.
