Use the conservation of energy to explain why the temperature of a well-insulated gas increases when it is compressed—say, by pushing down on a piston—whereas the temperature decreases when the gas expands. Show your reasoning.
In order to watch this solution you need to have a subscription.
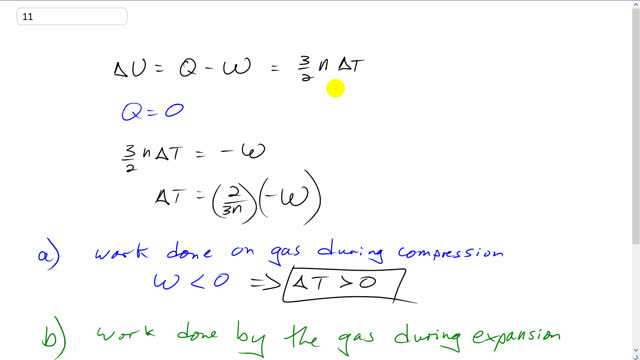
This is Giancoli Answers with Mr. Dychko. So the change in internal energy of the gas is the heat absorbed by the gas minus the work done by it. And this also equals three over two times the number of moles times the change in temperature. That's another equation for the change in internal energy. Now since the container is well insulated that means no heat is going to flow into or out of the gas so Q is zero. And this makes three over two n times change of temperature equal to negative W. And we can write this as change in temperature is two over three n times negative W but this isn't important. The only important thing is to notice that change in temperature is proportional to the negative of the work done by the gas. Now when there's work done on the gas, the work done by the gas is negative. And so if that means this… this number W is going to end up being a negative number. And so with this negative in front of it that’s gonna be two negatives which will combine to make a positive. So when there's work done on the gas, work done by the gas is negative and we'll end up with a rise in temperature Delta T to be positive. And whereas when there's work done by the gas during expansion that means the work that's done by the gas is positive. And that's going to lead to negative Delta T because Delta T will be negative of this positive number. So that's going to end up being a negative number in the end. And another way to think about it is when the gas does some work that's put energy into something else and that energy has to come from somewhere. It's not going to come from absorbing heat from its surroundings because the container is well insulated so there's no possibility for that to happen. Q is zero. And so this energy that goes into something else that it does work on has to come from the… The kinetic energy of the gas particles themselves and so those kinetic energy will have to decrease of all the particles. And that's another way to say that the temperature will have to decrease because the temperature is proportional to the kinetic energy of the particles.
