What is the change in entropy of 320 g of steam at when it is condensed to water at ?
In order to watch this solution you need to have a subscription.
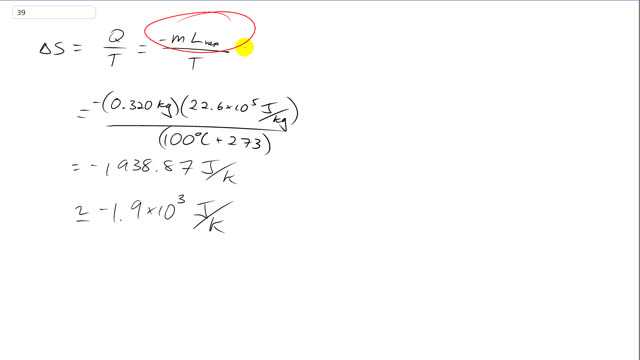
This is Giancoli answers with Mr. Dychko. The change in entropy of the steam is the amount of heat that the steam absorbs divided by its temperature. But the steam doesn't absorb any heat. It's actually a losing heat because it's condensing from vapor into liquid. So we put a negative sign in here to represent that it's losing some heat and the amount that it's going to lose is equal to the mass times the latent heat of vaporization. So this is the total amount of heat energy it'll give up just as part of its phase transition from vapor into liquid. All still at constant temperature 100 degrees Celsius. So we take a 0.32 kilograms, we have that negative in front, times 22.6 times ten to the five joules per kilogram latent heat of vaporization divided by 100 degrees Celsius converted to Kelvin by adding 273 for a total change in it to be of negative 1.9 times ten to three joules per Kelvin. So there is a reduction in entropy for the steam because it has gone from a disorderly vapor state into a more orderly liquid state. And the change in entropy of the universe however is always increasing in any process. But we're just talking about the change in entropy exclusively of the steam itself and that change is negative 1.9 times ten to three.
