In an engine, an almost ideal gas is compressed adiabatically to half its volume. In doing so, 2630 J of work is done on the gas.
- How much heat flows into or out of the gas?
- What is the change in internal energy of the gas?
- Does its temperature rise or fall?
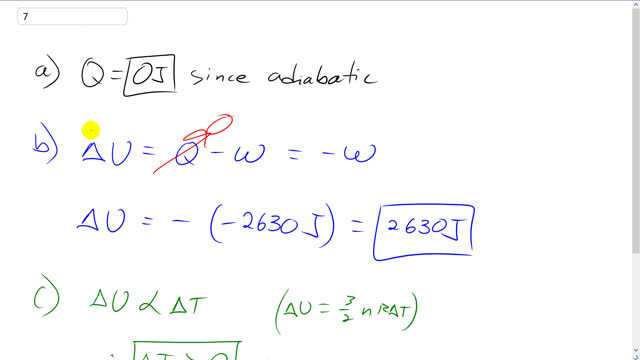
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. Because this process is adiabatic, that word means that there's no heat exchange. The gas doesn't absorb or give off any heat. Now change in internal energy is the heat absorbed by the gas minus the work done by it. But we already said that Q is zero because this process is adiabatic. So that term is gone and so we're just left with change in internal energy is negative of the work done by the gas but the work is done on the gas. And so that means this amount of work is negative because w itself represents the work done by the gas. So we have the minus sign from the formula and then negative because this is work done on the gas and w represents the work done by the gas. And so this negative and negative together makes positive 2630 joules change in internal energy. Now the change in internal energy is three over two n R times delta T. The change internal energy is proportional to the change in temperature or you could put it the other way around that change in temperature is proportional to a change in internal energy. So with a positive change in internal energy that means there's also a positive change in temperature so there is going to be a temperature rise as a result of this positive change in internal energy.
