How does the number of atoms in a 27.5-gram gold ring compare to the number in a silver ring of the same mass?
In order to watch this solution you need to have a subscription.
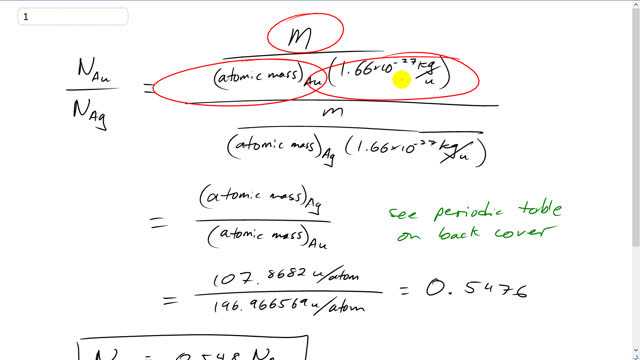
This is Giancoli Answers with Mr. Dychko. The number of gold atoms will be the mass of gold divided by the atomic mass of gold which is the number of atomic mass units per atom, multiplied by 1.66 times 10 to the minus 27 kilograms per atomic mass units so that the kilograms and between there and there cancel. And we see that the numerator is the same for the number of gold atoms as it is for the number of silver atoms because it's the same mass we're told. So, those m's are going to cancel and this conversion factor is going to cancel as well. And we can multiply top and bottom by this atomic mass of silver and that will cancel on the bottom and it will appear in the numerator of the top. So, what we're left with is just the atomic mass of silver divided by atomic mass of gold. And you can look in the periodic table in the back cover of your textbook to find the atomic mass of silver is 1.07 times 0.8682 atomic mass units per atom. And for gold it's 1.96 point... Or, sorry, I should say 196.966569 u per atom. And that gives 0.5476. So, the number of gold atoms is about half the number of silver atoms.
Hi, The process on this is a little confusing to me. In chemistry we had similar problems and we found them through a similar factor label method. I took 27.5 g of gold I took it through the mole, and divided by the atomic mass (199.9665). Then I multiplied by Avogadro number 6.022 *10^23. I did the same thing for the other then divided, however I got a very different answer. How come this way does not work? Also where did you get the 1.66*10^-27 kg/u?
Thank you!
Greg.
Hi Greg, thank you for your question. I think the difference between what I've done here and what is common in chemistry is that here I've used "atomic mass", whereas in chemistry "molar mass" is more common. When looking at the periodic table of elements, the mass given for each element can actually be interpreted either way. For gold, you can say it has an atomic mass (technically, this is a "relative atomic mass" which takes an average of all naturally occurring isotopes weighted by how common each isotope is) of or you can read it as , depending on your preference. Since I went with u/atom, I needed a conversion factor to turn the atomic mass unit (that's the "u") into kilograms, so that the "kg" in the numerator would cancel with "kg" in the denominator. The is the number you asked about: , which is just a constant you can look up in the front cover of the textbook under the section "Fundamental Constants" and it tells you how many kilograms are in one atomic mass unit.
I can't really say why your approach didn't work since I don't understand what you mean by "I took it through the mole". In any case, the chemistry approach definitely works. If the video took that approach, the atomic mass would be replaced with "molar mass", which is the same number but just different units, and then divide it by Avogadro's number.
Probably the confusing thing about this question is having fractions within fractions. Perhaps I should have written it on one line by multiplying by the reciprocal of the denominator. When checking your work make sure you're putting things in the right place within the fractions.
Hope this helps,
Mr. Dychko
Not correct for Mastering Physics
We were looking for Manganese instead of gold on our question. Thank you, you were correct!
