- At atmospheric pressure, in what phases can exist?
- For what range of pressures and temperatures can be a liquid? Refer to Fig. 13–23.
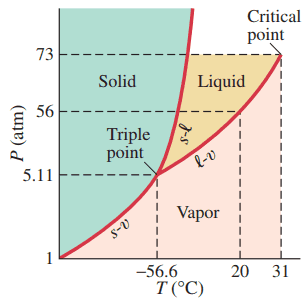
- solid or vapour
In order to watch this solution you need to have a subscription.
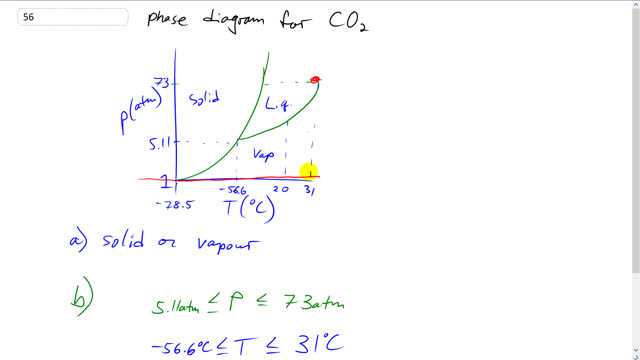
This is Giancoli Answers with Mr. Dychko. This is the phase diagram for carbon dioxide. And at a pressure of 1 atmosphere, we can see that the phase of vapor is possible. Anywhere between 31 degrees Celsius and below, we have vapor. And this point here, I had to look on Wikipedia to find this temperature here, it's not shown in the textbook. But this is the temperature when carbon dioxide become solid, and it's called dry ice, at atmospheric pressure of, at a temperature of 78.5 degrees below 0 Celsius. So... Yeah. So, at 1 atmosphere we can have the solid at this temperature of negative 78.5. And if the temperature falls below negative 78.5, it's still gonna be a solid. Or it can be a vapor or it could be a gas if you want to get technical and distinguish between vapors and gases, it'll be a gas beyond 31 but it'll look the same. So, and then... For what range of pressure and temperature can it be a liquid. Well, it can be a liquid any pressure above 5.11 atmospheres and below or equal to 73 atmospheres of pressure. And as far as temperatures are concerned, anywhere below 31 degrees Celsius and above 56.6 negative degrees Celsius.
