- What is the average translational kinetic energy of a nitrogen molecule at STP?
- What is the total translational kinetic energy of 1.0 mol of molecules at ?
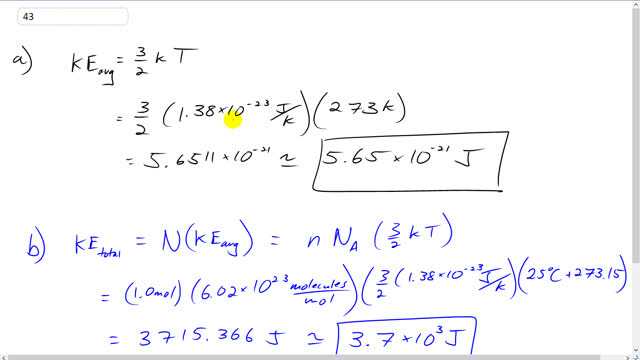
In order to watch this solution you need to have a subscription.
This is Giancoli Answers with Mr. Dychko. The average translational kinetic energy per molecule is 3 over 2 times Boltzmann constant times the absolute temperature in kelvin. So, it's 3 over 2 times 1.38 times 10 to the minus 23 joules per kelvin times 273 kelvin when you have standard temperature and pressure. And that gives 5.65 times 10 to the minus 21 joules per molecule of kinetic energy. The total kinetic energy of all the molecules is the number of molecules multiplied by the average kinetic energy of each molecule. And the number of molecules is the number of moles times Avogadro's number because Avogadro's number tells you the number of molecules per mole. And so 1 mole times this many molecules per mole gives me the number of molecules. So, it's 6.02 times 10 to the 23 molecules times 3 over 2 times Boltzmann’s constant, 1.38 times 10 to the minus 23 joules per kelvin, multiplied by the absolute temperature which is 25 degrees Celsius plus 273.15. And that give 3.7 times 10 to the 3 joules total kinetic energy of all the molecules put together.
